Short-Term Risk Factors for Bone Loss in Multiple Sclerosis: A Prospective Study and Literature Review
- PMID: 40304096
- PMCID: PMC12041888
- DOI: 10.1111/ene.70176
Short-Term Risk Factors for Bone Loss in Multiple Sclerosis: A Prospective Study and Literature Review
Abstract
Background: Reduced bone mass and increased osteoporosis risk are common in people with multiple sclerosis (pwMS). The aim of the study was to identify risk factors for short-term bone loss in MS.
Methods: This prospective study included 139 pwMS (ages 18-65). Baseline data included demographics, body-mass index, physical activity, smoking, menopause status, 25-hydroxy vitamin D levels, and history of glucocorticoid use. Bone mineral density (BMD) was measured at baseline and after 2 years using dual-energy X-ray absorptiometry (DXA) for the lumbar spine and hip. Disability worsening was assessed by the Expanded Disability Status Scale (EDSS). Additionally, a literature review was conducted on longitudinal data regarding BMD in MS.
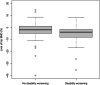
Results: Over the 2-year follow-up period, significant BMD loss was observed in the hip (baseline g/cm2: median 0.898; IQR 0.808-1.014; 2-year follow-up: 0.882; 0.784-1.01; p < 0.001), but not in the lumbar spine. Overall, 101 (73.1%) experienced hip BMD loss, with a median decrease of 3.5%. Patients with disability worsening had an approximately 7-times higher risk of bone loss compared to those without disability worsening (p = 0.013). PwMS with fractures during the follow-up period had significantly lower hip BMD (0.760, 0.546-0.890 vs. 0.909, 0.828-1.015; p = 0.024), a higher EDSS score (4.4, 2.8-5.8 vs. 2.0, 1.0-4.0 vs. p = 0.026), and were older (59, 46-62 vs. 47, 37-54; p = 0.030) compared to those without fractures.
Conclusion: Disability worsening was identified as a risk factor for BMD loss. These findings underscore the need for active monitoring of pwMS with disability worsening to prevent bone loss and, thus, to reduce fracture risk.
Keywords: bone fracture; bone mineral density; disability; multiple sclerosis; osteoporosis.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
A.Z. has participated in meetings sponsored by, received speaking honoraria or travel funding from Biogen, Merck, Novartis, Sanofi‐Genzyme, Janssen, Bristol Myers Squibb, and Teva. H.H. has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Bristol Myers Squibb, Horizon, Janssen, Merck, Novartis, Sanofi‐Genzyme, Siemens, Teva, and received honoraria for acting as a consultant for A‐med, Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi‐Genzyme, and Teva. J.W. has nothing to disclose. R.B. has participated in meetings sponsored by or received travel grants from Novartis, Janssen‐Cilag, Merck, and Sanofi‐Genzyme. Received honoraria from Janssen‐Cilag and Biogen. K.B. has participated in meetings sponsored by and received travel funding or speaker honoraria from Roche, Teva, Merck, Biogen, Sanofi, and Novartis. He is an associate editor of Frontiers in Immunology/Neurology, Section Multiple Sclerosis and Neuroimmunology. M.A. received speaker honoraria, honoraria for consulting and/or travel grants from Biogen, Novartis, Merck, Sanofi, Horizon Therapeutics/Amgen, and Zentiva. M.S. has participated in meetings sponsored by or received travel grants from Novartis, Sanofi‐Genzyme, and Amgen. G.B. has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Janssen, Lilly, Medwhizz, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva, and received honoraria for consulting Adivo Associates, Biogen, Celgene/BMS, Janssen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. He serves on the Executive Committee of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). A.S.K. has nothing to disclose. A.G. has nothing to disclose. B.W. has nothing to disclose. A.T. has nothing to disclose. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Amgen, Allergan, Bayer, Biogen, Bionorica, BMS, Genesis, GSK, GW/Jazz Pharma, Horizon, Janssen, MedDay, Merck, Neuraxpharma, Novartis, Octapharma, Roche, Sandoz, Sanofi, Teva, TG Therapeutics, and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS, Merck, Novartis, Roche, Sanofi, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi, and Teva. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, A‐Med, Amgen/Horizon, Biogen, BMS, Sanofi, Janssen, Laurea Group, Medwhizz, Merck, Novartis Pharma, Neuraxpharm, Roche, Sandoz, and Teva. He is section editor of the MSARD Journal (Multiple Sclerosis and Related Disorders) and review editor of Frontiers in Neurology. F.D.P. has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations), or travel funding from Amgen, Bayer, Biogen, Celgene, BMS, Horizon, Merck, Novartis, Sanofi‐Genzyme, Teva, and Roche.
Figures
Similar articles
-
Determinants of low bone mineral density in people with multiple sclerosis: Role of physical activity.Mult Scler Relat Disord. 2020 Feb;38:101864. doi: 10.1016/j.msard.2019.101864. Epub 2019 Nov 23. Mult Scler Relat Disord. 2020. PMID: 31801106
-
Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2006;6(20):1-180. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074491 Free PMC article.
-
Preoperative bone health assessment and optimization in spine surgery.Neurosurg Focus. 2020 Aug;49(2):E2. doi: 10.3171/2020.5.FOCUS20255. Neurosurg Focus. 2020. PMID: 32738805
-
Bone health in multiple sclerosis.Osteoporos Int. 2011 Dec;22(12):2935-49. doi: 10.1007/s00198-011-1644-8. Epub 2011 May 21. Osteoporos Int. 2011. PMID: 21604009 Review.
-
Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice.J Clin Densitom. 2017 Jul-Sep;20(3):334-345. doi: 10.1016/j.jocd.2017.06.019. Epub 2017 Jul 19. J Clin Densitom. 2017. PMID: 28734710 Review.
References
-
- Yazdan Panah M., Vaheb S., Moases Ghaffary E., Shaygannejad V., Zabeti A., and Mirmosayyeb O., “Bone Loss and Fracture in People With Multiple Sclerosis: A Systematic Review and Meta‐Analysis,” Multiple Sclerosis and Related Disorders 90 (2024): 105773. - PubMed
-
- Moen S. M., Celius E. G., Sandvik L., Nordsletten L., Eriksen E. F., and Holmøy T., “Low Bone Mass in Newly Diagnosed Multiple Sclerosis and Clinically Isolated Syndrome,” Neurology 77, no. 2 (2011): 151–157. - PubMed
-
- Nieves J., Cosman F., Herbert J., Shen V., and Lindsay R., “High Prevalence of Vitamin D Deficiency and Reduced Bone Mass in Multiple Sclerosis,” Neurology 44, no. 9 (1994): 1687–1692. - PubMed
-
- Olsson A., Oturai D. B., Sørensen P. S., Oturai P. S., and Oturai A. B., “Short‐Term, High‐Dose Glucocorticoid Treatment Does Not Contribute to Reduced Bone Mineral Density in Patients With Multiple Sclerosis,” Multiple Sclerosis 21, no. 12 (2015): 1557–1565. - PubMed
-
- Sioka C., Fotopoulos A., Papakonstantinou S., et al., “The Effect of Menarche Age, Parity and Lactation on Bone Mineral Density in Premenopausal Ambulatory Multiple Sclerosis Patients,” Multiple Sclerosis and Related Disorders 4, no. 4 (2015): 287–290. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical