Glecirasib, a Potent and Selective Covalent KRAS G12C Inhibitor Exhibiting Synergism with Cetuximab or SHP2 Inhibitor JAB-3312
- PMID: 40304209
- PMCID: PMC12076188
- DOI: 10.1158/2767-9764.CRC-25-0001
Glecirasib, a Potent and Selective Covalent KRAS G12C Inhibitor Exhibiting Synergism with Cetuximab or SHP2 Inhibitor JAB-3312
Abstract
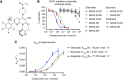
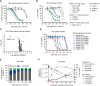
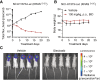
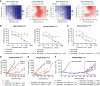
Glecirasib potently and selectively inhibits KRAS G12C and reduces ERK and AKT phosphorylation in KRAS G12C-mutant cancer cells, further inducing cell-cycle arrest and apoptosis. Glecirasib monotherapy leads to tumor regression in KRAS G12C-mutant animal models and shows synergistic effects with cetuximab or JAB-3312 (sitneprotafib).
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P. Wang reports a patent to WO2025036475A1 issued and a patent to WO2021121367A1 issued and being an employee of Jacobio Pharma. X. Sun reports being an employee of Jacobio Pharma. X. He reports being employee of Jacobio Pharma. D. Kang reports a patent to WO2025036475A1 issued and a patent to WO2018172984A1 issued and being an employee of Jacobio Pharma. X. Liu reports being employee of Jacobio Pharma. D. Liu reports a patent to WO2021121367A1 issued and being an employee of Jacobio Pharma. A. Li reports a patent to WO2025036475A1 issued, a patent to WO2022237815A1 issued, and a patent to WO2021121367A1 issued and being an employee of Jacobio Pharma. G. Yang reports being an employee of Jacobio Pharma. Y. Lin reports being a current employee of Jacobio Pharma. S. Li reports a patent to WO2022237815A1 issued and a patent to WO2021121367A1 issued and being an employee of Jacobio Pharma. Yinxiang Wang reports a patent to WO2025036470A1 issued and a patent to WO2025036475A1 issued and being an employee and shareholder of Jacobio Pharma. Yanping Wang reports a patent to WO2025036470A1 issued and a patent to WO2025036475A1 issued and being an employee of Jacobio Pharma.
Figures

Similar articles
-
Combinations with Allosteric SHP2 Inhibitor TNO155 to Block Receptor Tyrosine Kinase Signaling.Clin Cancer Res. 2021 Jan 1;27(1):342-354. doi: 10.1158/1078-0432.CCR-20-2718. Epub 2020 Oct 12. Clin Cancer Res. 2021. PMID: 33046519
-
JAB-3312, a Potent Allosteric SHP2 Inhibitor That Enhances the Efficacy of RTK/RAS/MAPK and PD-1 Blockade Therapies.Clin Cancer Res. 2025 Jul 15;31(14):3019-3032. doi: 10.1158/1078-0432.CCR-24-3691. Clin Cancer Res. 2025. PMID: 40333694
-
Co-targeting SOS1 enhances the antitumor effects of KRASG12C inhibitors by addressing intrinsic and acquired resistance.Nat Cancer. 2024 Sep;5(9):1352-1370. doi: 10.1038/s43018-024-00800-6. Epub 2024 Aug 5. Nat Cancer. 2024. PMID: 39103541 Free PMC article.
-
Targeting Krasg12c -mutant cancer with a mutation-specific inhibitor.J Intern Med. 2020 Aug;288(2):183-191. doi: 10.1111/joim.13057. Epub 2020 Apr 7. J Intern Med. 2020. PMID: 32176377 Review.
-
Small molecules for Kirsten rat sarcoma viral oncogene homolog mutant cancers: Past, present, and future.Eur J Pharmacol. 2025 Jun 5;996:177428. doi: 10.1016/j.ejphar.2025.177428. Epub 2025 Feb 28. Eur J Pharmacol. 2025. PMID: 40024323 Review.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. . Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229–63. - PubMed
-
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet 2021;398:535–54. - PubMed
-
- Swanton C, Govindan R. Clinical implications of genomic discoveries in lung cancer. N Engl J Med 2016;374:1864–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

