Accelerating Cleavage Activity of CRISPR-Cas13 System on a Microfluidic Chip for Rapid Detection of RNA
- PMID: 40304259
- PMCID: PMC12079638
- DOI: 10.1021/acs.analchem.5c00256
Accelerating Cleavage Activity of CRISPR-Cas13 System on a Microfluidic Chip for Rapid Detection of RNA
Abstract
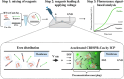
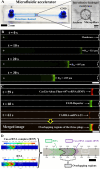
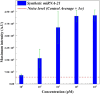
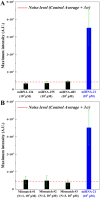
It is extremely advantageous to detect nucleic acid levels in the early phases of disease management; such early detection facilitates timely treatment, and it can prevent altogether certain cancers and infectious diseases. A simple, rapid, and versatile detection platform without enzymatic amplification for both short and long sequences would be highly desirable in this regard. Our study addresses this need by introducing IMACC, an
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Calin G. A.; Croce C. M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6 (11), 857–866. 10.1038/nrc1997. - DOI - PubMed
- Grubaugh N. D.; Ladner J. T.; Lemey P.; Pybus O. G.; Rambaut A.; Holmes E. C.; Andersen K. G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2019, 4 (1), 10–19. 10.1038/s41564-018-0296-2. - DOI - PMC - PubMed
- Siravegna G.; Marsoni S.; Siena S.; Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14 (9), 531–548. 10.1038/nrclinonc.2017.14. - DOI - PubMed
-
- Yang S.; Rothman R. E. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4 (6), 337–348. 10.1016/S1473-3099(04)01044-8. - DOI - PMC - PubMed
- Burki T. K. Testing for COVID-19. Lancet Respir. Med. 2020, 8 (7), E63–E64. 10.1016/S2213-2600(20)30247-2. - DOI - PMC - PubMed
- Weissleder R.; Lee H.; Ko J.; Pittet M. J. COVID-19 diagnostics in context. Sci. Transl. Med. 2020, 12 (546), eabc193110.1126/scitranslmed.abc1931. - DOI - PubMed
-
- Mahony J. B.; Blackhouse G.; Babwah J.; Smieja M.; Buracond S.; Chong S.; Ciccotelli W.; O’Shea T.; O’Shea T.; Alnakhli D.; Griffiths-Turner M. Cost Analysis of Multiplex PCR Testing for Diagnosing Respiratory Virus Infections. J. Clin. Microbiol. 2009, 47 (9), 2812–2817. 10.1128/JCM.00556-09. - DOI - PMC - PubMed
-
- Wang D. G.; Brewster J. D.; Paul M.; Tomasula P. M. Two Methods for Increased Specificity and Sensitivity in Loop-Mediated Isothermal Amplification. Molecules 2015, 20 (4), 6048–6059. 10.3390/molecules20046048. - DOI - PMC - PubMed
- Phillips E. A.; Moehling T. J.; Bhadra S.; Ellington A. D.; Linnes J. C. Strand Displacement Probes Combined with Isothermal Nucleic Acid Amplification for Instrument-Free Detection from Complex Samples. Anal. Chem. 2018, 90 (11), 6580–6586. 10.1021/acs.analchem.8b00269. - DOI - PMC - PubMed
-
- Yozwiak N. L.; Skewes-Cox P.; Stenglein M. D.; Balmaseda A.; Harris E.; DeRisi J. L. Virus Identification in Unknown Tropical Febrile Illness Cases Using Deep Sequencing. PLoS Neglect. Trop. Dis. 2012, 6 (2), e148510.1371/journal.pntd.0001485. - DOI - PMC - PubMed
- Wilson M. R.; Naccache S. N.; Samayoa E.; Biagtan M.; Bashir H.; Yu G. X.; Salamat S. M.; Somasekar S.; Federman S.; Miller S.; et al. Actionable Diagnosis of Neuroleptospirosis by Next-Generation Sequencing. New Engl. J. Med. 2014, 370 (25), 2408–2417. 10.1056/NEJMoa1401268. - DOI - PMC - PubMed
- Langelier C.; Zinter M. S.; Kalantar K.; Yanik G. A.; Christenson S.; O’Donovan B.; White C.; Wilson M.; Sapru A.; Dvorak C. C.; et al. Metagenomic Sequencing Detects Respiratory Pathogens in Hematopoietic Cellular Transplant Patients. Am. J. Respir. Crit. Care 2018, 197 (4), 524–528. 10.1164/rccm.201706-1097LE. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

