Physical activity changes during midlife link to brain integrity and amyloid burden
- PMID: 40304268
- PMCID: PMC12042120
- DOI: 10.1002/alz.70007
Physical activity changes during midlife link to brain integrity and amyloid burden
Abstract
Introduction: Evidence suggests that midlife physical activity may reduce Alzheimer's disease (AD) risk. In at-risk individuals, we investigated midlife physical activity changes in relation to AD-related pathologies.
Methods: We included 337 cognitively unimpaired adults with baseline and follow-up physical activity evaluations within 4.07 ± 0.84 years. We performed multiple regressions considering follow-up amyloid-PET burden and MRI-based medial temporal lobe cortical thickness as outcomes. Independent variables encompassed changes in adherence to World Health Organization (WHO)-recommended physical activity levels, activity amounts, and sedentary behavior (no activity reported).
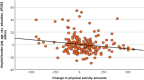
Results: Remaining sedentary was associated with lower cortical thickness compared to doing limited physical activity, maintaining adherence, or becoming adherent to WHO recommendations. Becoming adherent to recommendations was linked to lower amyloid burden compared to becoming non-adherent. Increased activity amounts showed a dose-dependent association with lower amyloid burden.
Discussion: Increasing physical activity and new adherence to WHO recommendations could be key objectives for preventive strategies during midlife.
Clinical trial registration information: Registered at Clinicaltrials.gov (identifier: NCT02485730).
Highlights: Boosting physical activity in midlife may have beneficial effects in preclinical AD. Physical activity increases relate to lower Aβ burden in a dose-dependent manner. Remaining sedentary links to lower cortical thickness in AD-vulnerable structures. New adherence to WHO-recommended physical activity levels may enhance brain health.
Keywords: Alzheimer's disease; amyloid‐β; cortical thickness; midlife physical activity; physical activity change; prevention.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
O.G‐R. has given lectures in symposia sponsored by Roche Diagnostics, and receives support for research (to the institution) from F‐ Hoffmann La Roche. MSC has given lectures in symposia sponsored by Roche Diagnostics, S.L.U, Roche Farma, S.A and Amirall. He has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and Grifols S.L. He was granted with a project funded by Roche Diagnostics International Ltd; payments were made to the institution (BBRC). He received in‐kind support for research (to the institution) from Roche Diagnostics International Ltd, Avid Radiopharmaceuticals, Inc., Eli Lilly and Janssen Research & Development. J.G.‐A. reports speaking and lecture fees from Chiesi España and AstraZeneca Pharmaceuticals LP, not related to the topic of this publication. M.A., P.A.‐D., E.P., M.G.‐P., M.S., C.D., K.F., J.D.G., G.S.‐B., and E.M.A.‐U. declare that they have no competing interests. Author disclosures are available in the supporting information.
Figures
References
-
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2011;7(3):280‐292. doi:10.1016/j.jalz.2011.03.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- ID 100010434/'la Caixa' Foundation
- LCF/PR/GN17/50300004/'la Caixa' Foundation
- AARG 2019-AARG-644641/ALZ/Alzheimer's Association/United States
- AARG 2019-AARG-644641-RAPID/ALZ/Alzheimer's Association/United States
- International Anonymous Charity
- TriBEKa-17-519007/TriBEKa Imaging Platform project
- 2021 SGR 00913/Universities and Research Secretariat, Ministry of Business and Knowledge of the Catalan Government
- PID2019-111514RA-I00/Ministry of Science and Innovation
- European Social Fund (ESF)
- JPND2022-138/EU Joint Programme-Neurodegenerative Disease Research
- No. 948677/European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme
- PI19/00155/Instituto de Salud Carlos III (ISCIII)
- European Union
- 847648/European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement
- CEX2018000806-S/MCIN/AEI/10.13039/501100011033
- Generalitat de Catalunya CERCA Program
- RYC2018-026053-I/Spanish Ministry of Science and Innovation - State Research Agency
- PRE2020-095827/the Spanish Ministry of Science and Innovation

