Quinol-Enedione Rearrangement
- PMID: 40304360
- PMCID: PMC12070464
- DOI: 10.1021/acs.orglett.5c01266
Quinol-Enedione Rearrangement
Abstract
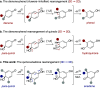
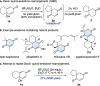
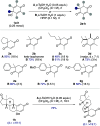
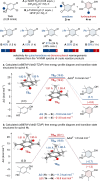
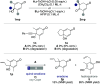
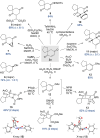
The quinol-enedione rearrangement enables the synthesis of 2-cyclohexene-1,4-diones from readily available para-quinol substrates. Building on sporadic early reports of this transformation, we have optimized the reaction conditions and systematically investigated its substrate scope. The utility of Brønsted acid-mediated reaction conditions for a variety of quinol derivatives, including those with substituted and unsubstituted migrating termini, is highlighted. Notably, kinetic selectivity between quinol-enedione and dienone-phenol rearrangements is demonstrated. The synthetic potential of the enedione products is showcased through a range of transformations, leading to the formation of complex polycyclic structures. These findings provide a valuable framework for recognizing and applying the quinol-enedione rearrangement in synthesis, while computational studies offer valuable insights into its mechanistic underpinnings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Andreocci A. About two new isomers of santonin and santonin acid. Gazz. Chim. Ital. 1893, 23, 468–476.
- Auwers K. V.; Ziegler K. Hydrocarbons of the Semibenzene Group. Justus Liebigs Ann. Chem. 1921, 425, 217–280. 10.1002/jlac.19214250302. - DOI
- Clemo G. R.; Haworth R. D.; Walton E. Constitution of santonin. II. Synthesis of racemic desmotroposantonin. J. Chem. Soc. 1930, 1110–1115. 10.1039/JR9300001110. - DOI
- Wilds A. L.; Djerassi C. Dienone-phenol rearrangement applied to chrysene derivatives. The synthesis of 3-hydroxy-1-methylchrysene and related compounds. J. Am. Chem. Soc. 1946, 68, 1715–1719. 10.1021/ja01213a010. - DOI - PubMed
-
- For a useful overview, see: Kurti L.; Czako B.. Dienone–Phenol Rearrangement. In Strategic Applications of Named Reactions in Organic Synthesis; Kurti L., Czako B., Eds.; Elsevier Academic Press, 2005; pp 142–143.
-
For specific examples, see:
- Hart D. J.; Kim A.; Krishnamurthy R.; Merriman G. H.; Waltos A. M. Synthesis of 6H-dibenzo[b,d]pyran-6-ones via dienone-phenol rearrangements of spiro[2,5-cyclohexadiene-1,1’(3′H)-isobenzofuran]-3′-ones. Tetrahedron 1992, 48, 8179–8188. 10.1016/S0040-4020(01)80487-7. - DOI
- Parker K. A.; Koh Y. -h. Methodology for the Regiospecific Synthesis of Bis C-Aryl Glycosides. Models for Kidamycins. J. Am. Chem. Soc. 1994, 116, 11149–11150. 10.1021/ja00103a037. - DOI
- Guo Z.; Schultz A. G. Preparation and Photochemical Rearrangements of 2-Phenyl-2,5-cyclohexadien-1-ones. An Efficient Route to Highly Substituted Phenols. Org. Lett. 2001, 3, 1177–1180. 10.1021/ol015637h. - DOI - PubMed
-
- Davis B. R.; Woodgate P. D. Dienone–Phenol Type Rearrangements. Part III. para-Quinols. J. Chem. Soc. C 1968, 0, 712–715. 10.1039/J39680000712. - DOI
-
- Burkinshaw G. F.; Davis B. R.; Woodgate P. D.; Hodges R. The Isolation of a Spiran in the Acid-catalysed Rearrangement of a Bicyclic Cyclohexadienone. Chem. Commun. 1968, 528.10.1039/c19680000528. - DOI
- Burkinshaw G. F.; Davis B. R.; Hutchinson E. G.; Woodgate P. D.; Hodges R. The synthesis and acid-catalysed rearrangements of 4-hydroxycyclohexa-2,5-dienones. J. Chem. Soc. C 1971, 3002–3006. 10.1039/j39710003002. - DOI
- Berger S.; Henes G.; Rieker A.; et al. Baseninduzierte Acyloin-Umlagerung sterisch gehinderter p-Chinole. Chem. Ber. 1976, 109, 1530–1548. 10.1002/cber.19761090439. - DOI
- Planas A.; Tomás J.; Bonet J.-J. Spiran isolation in the dienone-phenol rearrangement of steroidal p-quinols. Tetrahedron Lett. 1987, 28, 471–474. 10.1016/S0040-4039(00)95759-9. - DOI
- Uno H.; Yayama A.; Suzuki H. 1,2-Migration of Perfluoroalkyl Groups in Anionotropic Rearrangement. The Acyloin Rearrangement of 4-Perfluoroalkyl-4-quinols. Chem. Lett. 1991, 20, 1165–1168. 10.1246/cl.1991.1165. - DOI
- Uno H.; Yayama A.; Suzuki H. Perfluoroalkyl Migration in the Rearrangement of 4-Perfluoroalkyl-4-quinols. Tetrahedron 1992, 48, 8353–8368. 10.1016/S0040-4020(01)86584-4. - DOI
LinkOut - more resources
Full Text Sources

