ClpB enhances thermotolerance in Campylobacter jejuni through protein disaggregation independent of DnaK
- PMID: 40304467
- PMCID: PMC12131804
- DOI: 10.1128/spectrum.02293-24
ClpB enhances thermotolerance in Campylobacter jejuni through protein disaggregation independent of DnaK
Erratum in
-
Erratum for Hur et al., "ClpB enhances thermotolerance in Campylobacter jejuni through protein disaggregation independent of DnaK".Microbiol Spectr. 2025 Nov 4;13(11):e0289825. doi: 10.1128/spectrum.02898-25. Epub 2025 Oct 7. Microbiol Spectr. 2025. PMID: 41055430 Free PMC article. No abstract available.
Abstract
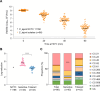
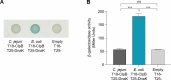
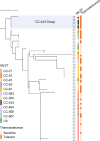
Campylobacter jejuni is a leading cause of foodborne infections worldwide and primarily transmitted to humans through the consumption of contaminated poultry meat. To enhance Campylobacter-associated food safety, it is critical to understand how C. jejuni survives during the thermal processing of poultry products. In this study, we monitored the survival of 86 C. jejuni strains during heat treatment and observed that some strains exhibited elevated heat tolerance. Notably, multilocus sequence typing clonal complex (CC)-443 and CC-607 were dominant among heat-tolerant strains, while the CC-21 strains were mostly heat-sensitive, indicating phylogenetic association with thermotolerance. We also investigated the function of heat shock chaperones in the thermotolerance of C. jejuni. Among several knockout mutants of heat shock chaperones, a mutant lacking clpB exhibited significantly lower survival than the wild type under heat treatment. Moreover, we observed a significantly higher accumulation of protein aggregates in the absence of ClpB, demonstrating that ClpB functions as a disaggregase during heat exposure. Additionally, ClpB from the heat-tolerant CC-443 group possessed distinct amino acid substitutions in the functional nucleotide-binding domain compared to ClpB in other CC groups. Interestingly, despite the well-known interaction of these proteins in many other bacteria, a two-hybrid assay demonstrated that ClpB of C. jejuni does not bind to DnaK, suggesting that C. jejuni may have a distinct mechanism for protein disaggregation and stress tolerance. Our findings demonstrate that ClpB plays a crucial role in the thermotolerance of C. jejuni through unique protein disaggregation mechanisms during poultry processing.IMPORTANCEThis study unveils a distinctive mechanism of thermotolerance involving protein disaggregation in Campylobacter jejuni, a major foodborne pathogen. Understanding C. jejuni's ability to withstand heat stress is crucial for comprehending the occurrence of Campylobacter infections resulting from the consumption of contaminated poultry meat. Our research elucidates the roles of heat shock proteins, particularly ClpB, in the thermotolerance of C. jejuni. These findings significantly contribute to our fundamental understanding of bacterial physiology related to stress tolerance, which has important implications for public health and food safety.
Keywords: Campylobacter; ClpB; multilocus sequence typing (MLST); protein disaggregation; thermotolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- European Food Safety Authority . 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008. EFS2 8. doi: 10.2903/j.efsa.2010.1503 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

