Microbial and metabolic profiles associated with HPV infection and cervical intraepithelial neoplasia: a multi-omics study
- PMID: 40304477
- PMCID: PMC12131762
- DOI: 10.1128/spectrum.00192-25
Microbial and metabolic profiles associated with HPV infection and cervical intraepithelial neoplasia: a multi-omics study
Abstract
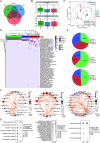
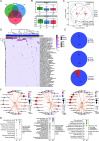
Cervical cancer is the most common malignancy of the female reproductive system, with the incidence of human papillomavirus (HPV) being a crucial factor in its pathogenesis. Emerging evidence indicates that cervicovaginal microbiota may influence HPV persistence and cervical intraepithelial neoplasia (CIN). However, the interplay between cervicovaginal and cervical tissue microbiomes and their association with HPV infection and CIN remains poorly understood. In this cross-sectional study, we analyzed the microbiota profiles of cervicovaginal and cervical tissue via five-region 16S rRNA gene metabarcoding, along with cervicovaginal metabolites, including short-chain fatty acids (SCFAs) and non-targeted metabolomic data, from 94 women. Key species, particularly Lacticaseibacillus and various anaerobes, are vital components of the microbiota found in both cervicovaginal secretions and cervical tissue, despite notable differences in microbial composition. The CIN group exhibited significant differences in microbial diversity and composition compared to the control groups, with key species such as Lacticaseibacillus iners and Prevotella bivia associated with HPV status and CIN progression. Metabolomic analysis revealed alterations in glycerophospholipid metabolism, but not in SCFAs, with correlations observed between metabolites and HPV status. Notable associations, including P. bivia-PE(18:1/0:0)-HPV and Fusobacterium periodonticum-PI(40:6)-HPV, were identified. Our findings emphasize the critical roles of cervicovaginal and cervical tissue microbiomes in HPV infection and CIN development, highlighting specific microbial species and metabolic pathways for early detection and therapeutic targets.
Importance: Cervical cancer is the most prevalent malignancy in the female reproductive system, with human papillomavirus (HPV) persistency being a critical factor in its pathogenesis. This study highlights the significant yet often overlooked role of cervicovaginal secretion and cervical tissue microbiota in influencing HPV infection and the progression of cervical intraepithelial neoplasia (CIN). By employing a multi-omics approach, we elucidated distinct microbiota profiles in cervical tissues compared to cervicovaginal secretions, revealing a complex interplay between specific bacterial species (notably Lacticaseibacillus and anaerobes) and metabolomic changes associated with glycerophospholipid metabolism. Our findings address a significant gap in understanding the interplay between cervicovaginal secretion and cervical intratissue microbiomes, HPV infection, and CIN.
Keywords: HPV; cervical intraepithelial neoplasia; metabolome; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

