Novel murine model of human astrovirus infection reveals cardiovascular tropism
- PMID: 40304490
- PMCID: PMC12090817
- DOI: 10.1128/jvi.00240-25
Novel murine model of human astrovirus infection reveals cardiovascular tropism
Abstract
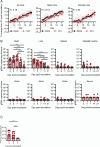
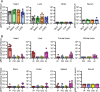
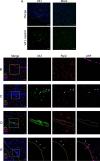
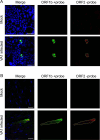
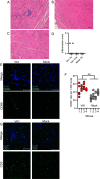
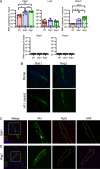
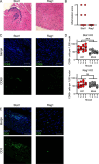
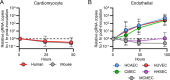
Astroviruses are a common cause of gastrointestinal disease in humans and have been linked to fatal cases of encephalitis. A major barrier to the study of human-infecting astroviruses is the lack of an in vivo model as previous attempts failed to identify a host that supports viral replication. We describe a novel murine model of infection using astrovirus VA1/HMO-C (VA1), an astrovirus with high seroprevalence in humans. VA1 is cardiotropic, and viral RNA levels peak in the heart tissue 7 days post-inoculation in multiple different murine genetic backgrounds. Infectious VA1 particles could be recovered from heart tissue 3 and 5 days post-inoculation. Viral capsid was detected intracellularly in the heart tissue by immunostaining, and viral RNA was detected in cardiac myocytes, endocardium, and endothelial cells based on fluorescent in situ hybridization and confocal microscopy. Histologically, we identified inflammatory infiltrates consistent with myocarditis in some mice, with viral RNA colocalizing with the infiltrates. These foci contained CD3 +T cells and CD68 +macrophages. Viral RNA levels increased by >10 fold in the heart tissue or serum samples from Rag1 or Stat1 knockout mice, demonstrating the role of both adaptive and innate immunity in the response to VA1 infection. Based on the in vivo tropisms, we tested cardiac-derived primary cells and determined that VA1 can replicate in primary human cardiac endothelial cells, suggesting a novel cardiovascular tropism in human cells. This novel in vivo model of a human-infecting astrovirus enables further characterization of the host immune response and reveals a new cardiovascular tropism of astroviruses.
Importance: Astroviruses routinely cause infections in humans; however, few methods were available to study these viruses. Here, we describe the first animal system to study human-infecting astroviruses by using mice. We demonstrate that mice are susceptible to astrovirus VA1, a strain that commonly infects humans and has been linked to fatal brain infections. The virus infects the heart tissue and is associated with inflammation. When mice with impaired immune systems were infected with VA1, they were found to have higher amounts of the virus in their hearts and blood. We found that VA1 can infect cells from human blood vessels of the heart, which is associated with human health. This model will enable us to better understand how astroviruses cause disease and how the immune system responds to infection. Our findings also suggest that astroviruses could be linked to cardiovascular diseases, including in humans.
Keywords: animal models; astrovirus; myocarditis; tropism; viral immunity; virology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

