Spike proteins of coronaviruses activate mast cells for degranulation via stimulating Src/PI3K/AKT/Ca2+ intracellular signaling cascade
- PMID: 40304504
- PMCID: PMC12090780
- DOI: 10.1128/jvi.00078-25
Spike proteins of coronaviruses activate mast cells for degranulation via stimulating Src/PI3K/AKT/Ca2+ intracellular signaling cascade
Abstract
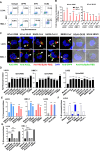
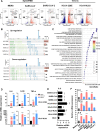
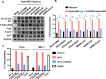
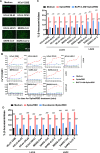
Mast cells (MCs) are strategically located at the interface between host and environment. The non-allergic functions of MCs in immunosurveillance against pathogens have been recently underscored. However, the activation of MCs by pathogens may beneficially or detrimentally regulate immune inflammation to combat or promote pathogen invasion. We and others have conclusively demonstrated that MCs serve as a crucial mediator in the induction of hyperinflammation initiated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to substantial tissue damage across multiple organs in murine and nonhuman primate models. Whereas the precise mechanism underlying virus-induced MC activation and degranulation remains largely elusive, our previous findings have indicated that the binding of the Spike proteins to cellular receptors is sufficient to elicit MC activation for rapid degranulation. This study aims to corroborate the ubiquity of coronavirus-induced MC degranulation and elucidate the intracellular signaling pathways that mediate the activation of MCs upon Spike protein binding to the cellular receptors. Our transcriptome analysis revealed MC activation upon the stimulations with a range of Spike/RBD proteins and viral particles of coronavirus. Notably, the interaction between these Spike/RBD proteins and cellular receptors triggered the activation of src kinase, a member of Src Family Kinases (SFKs). This activation, in turn, stimulated the PI3K/AKT signaling pathway, resulting in an accumulation of intracellular calcium ions. These calcium ions subsequently facilitated microtubule-dependent granule transport, ultimately promoting MC degranulation. In summary, this study elucidates the mechanism underlying virus-triggered activation of MCs and has the potential to aid in the development of MC-targeted antiviral therapeutic strategies.
Importance: The activation and degranulation of mast cells (MCs), triggered by a variety of viruses, are intricately linked to viral pathogenesis. However, the precise mechanism underlying virus-induced MC degranulation remains largely unknown. In this study, we demonstrate the ubiquity of coronavirus-induced MC degranulation and investigate the intracellular signaling pathways that mediate this process. We reveal that the binding of Spike proteins and cellular receptors is sufficient to elicit MC activation for rapid degranulation. This binding triggers the activation of src kinase and the downstream PI3K/AKT cellular signaling pathway, resulting in an accumulation of intracellular calcium ions. These calcium ions subsequently facilitate microtubule-dependent granule transport, ultimately promoting MC degranulation. This study elucidates the mechanism underlying virus-triggered activation of MCs and has the potential to aid in the development of MC-targeted antiviral therapeutic strategies.
Keywords: coronavirus; degranulation; mast cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury.Signal Transduct Target Ther. 2021 Dec 17;6(1):428. doi: 10.1038/s41392-021-00849-0. Signal Transduct Target Ther. 2021. PMID: 34921131 Free PMC article.
-
Mast cell degranulation-triggered by SARS-CoV-2 induces tracheal-bronchial epithelial inflammation and injury.Virol Sin. 2024 Apr;39(2):309-318. doi: 10.1016/j.virs.2024.03.001. Epub 2024 Mar 6. Virol Sin. 2024. PMID: 38458399 Free PMC article.
-
MrgprX2 regulates mast cell degranulation through PI3K/AKT and PLCγ signaling in pseudo-allergic reactions.Int Immunopharmacol. 2022 Jan;102:108389. doi: 10.1016/j.intimp.2021.108389. Epub 2021 Dec 15. Int Immunopharmacol. 2022. PMID: 34920312
-
Intracellular signaling pathways in IgE-dependent mast cell activation.Arch Immunol Ther Exp (Warsz). 2006 Nov-Dec;54(6):393-401. doi: 10.1007/s00005-006-0049-4. Epub 2006 Nov 21. Arch Immunol Ther Exp (Warsz). 2006. PMID: 17122878 Review.
-
[Elucidation of Mast Cell Activation Mechanism Mediated by Purinergic Signaling].Yakugaku Zasshi. 2021;141(9):1057-1061. doi: 10.1248/yakushi.21-00113. Yakugaku Zasshi. 2021. PMID: 34471006 Review. Japanese.
References
-
- Boziki M, Theotokis P, Kesidou E, Nella M, Bakirtzis C, Karafoulidou E, Tzitiridou-Chatzopoulou M, Doulberis M, Kazakos E, Deretzi G, Grigoriadis N, Kountouras J. 2024. Impact of mast cell activation on neurodegeneration: a potential role for gut-brain axis and Helicobacter pylori infection. Neurol Int 16:1750–1778. doi:10.3390/neurolint16060127 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

