Rhizosphere-colonizing bacteria persist in the protist microbiome
- PMID: 40304530
- PMCID: PMC12108058
- DOI: 10.1128/msphere.00037-25
Rhizosphere-colonizing bacteria persist in the protist microbiome
Abstract
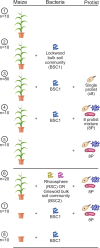
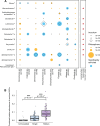
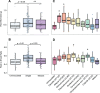
Soils contain diverse predatory protists that affect the abundance and behavior of rhizosphere bacteria, including bacteria that may benefit plant health. Protists harbor their own bacterial microbiomes, and we previously observed that plants inoculated with protists harbored rhizosphere bacteria similar to those in the protist inoculum. To determine how protist microbiomes affect the rhizosphere, we profiled the bacteria of eight diverse rhizosphere protist isolates after 2 years of laboratory culture. We then compared the protist culture microbiomes to maize rhizosphere communities 6 weeks after protist inoculation. Introduction of protists enriched 13 protist-associated bacterial amplicon sequence variants (ASVs) in the rhizosphere, which comprised ~10% of the rhizosphere bacterial community. Additional bacterial ASVs ranked highly in abundance in both rhizosphere (top 100) and protist (top 20) microbiomes; together, a median 47% of the protist microbiome was enriched or in high rank abundance in the rhizosphere. Inoculation with three out of eight protist cultures positively affected root biomass traits, but a protist mixture had no effect, indicating that the impact of protist-associated bacteria on plant growth is context dependent. Isolates of protist-associated bacteria had both positive and negative effects on protist growth in culture, suggesting that the bacteria use multiple strategies to survive in proximity to predators. This study demonstrates that even after long-term laboratory culture, rhizosphere protist cultures host bacteria that can colonize the rhizosphere of maize. The findings also identify diverse groups of rhizosphere-colonizing bacteria that persist among protist predators, which suggests that these bacteria could associate with or benefit from protists in the soil.
Importance: Understanding the impact of predatory protists on the plant microbiome will be essential to deploy protists in sustainable agriculture. This study shows that eight rhizosphere protist isolates hosted diverse and distinct bacterial communities and that a large proportion of these bacteria could be found colonizing the maize root environment 6 weeks after protists were inoculated onto seedlings. This study demonstrates that certain bacteria from the maize rhizosphere can persist for years in protist cultures and retain the ability to colonize rhizosphere soil, suggesting that protists might support the survival of these rhizosphere bacteria in the absence of the plant.
Keywords: Colpoda; maize; microbiome; protist; rhizosphere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Martins SJ, Taerum SJ, Triplett L, Emerson JB, Zasada I, de Toledo BF, Kovac J, Martin K, Bull CT. 2022. Predators of soil bacteria in plant and human health. Phytobiomes J 6:184–200. doi:10.1094/PBIOMES-11-21-0073-RVW - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
