Swiss Quality of Life and Health Care Impact Assessment in a Real-World Erenumab-Treated Migraine Population: Results over 2 years
- PMID: 40304557
- PMCID: PMC12266637
- DOI: 10.1111/head.14943
Swiss Quality of Life and Health Care Impact Assessment in a Real-World Erenumab-Treated Migraine Population: Results over 2 years
Abstract
Objectives/background: This prospective, multicenter, noninterventional cohort study evaluated the impact of erenumab on the quality of life and migraine-related impairment of adult patients with migraine, as well as the drug's tolerability in a real-world setting over 2 years. Erenumab, a fully human monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) receptor, was licensed in Switzerland for prophylactic treatment of adult patients with migraine in July 2018.
Methods: Adult patients with chronic or episodic migraine who received erenumab treatment as per Swiss label were enrolled from 19 sites across Switzerland between February 2019 and November 2022. Inclusion criteria were defined as the following: a migraine diagnosis per International Classification of Headache Disorders, 3rd edition, patient written informed consent, and adherence to Swiss label guidelines. Exclusion criteria were concomitant use of other investigational drugs or prior treatment with erenumab or any CGRP (receptor)-based therapy. Retrospective and prospective data from patients were collected over 24 months using patient-reported outcome questionnaires (Headache Impact Test-6 [HIT-6], modified Migraine Disability Assessment [mMIDAS], Impact of Migraine on Partners and Adolescent Children [IMPAC]), patient diaries, and medical charts to track migraine days, medication use, and health care utilization. Swiss regulations required patients to maintain migraine diaries 3 months before and during erenumab therapy to record migraine and acute medication days.
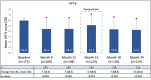
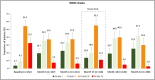
Results: A total of 173 patients (84.9% females) were enrolled who had an average age of 44.2 years. Of these, 54.3% were diagnosed with episodic migraine and 45.7% with chronic migraine. At baseline, the participants scored 65.9 ± 4.9 (mean ± SD) on HIT-6. A total of 85.5% had grade III-IV on the mMIDAS and 87% had grade III-IV in IMPAC. Patients reported 16.5 ± 7.2 monthly migraine days and 11.5 ± 7.0 acute migraine-specific medication days per month. After 24 months, the mean HIT-6 score had decreased by 8.1 ± 8.6, mean mMIDAS by 16.6 ± 21.2, monthly migraine days by 8.8 ± 7.6, and mean acute migraine-specific medication days by 5.2 ± 6.8 (all p < 0.001). Reductions of IMPAC by 6.5 ± 6.6 (p < 0.001) showed the beneficial impact of erenumab treatment on the patients' family burden attributed to migraine. Overall, 84 of 173 patients (48.6%) had at least one adverse event and 61 of 173 patients (35.3%) had at least one adverse event related to study drug. The safety profile was in line with the pivotal studies, and no serious adverse events were regarded as being related to erenumab.
Conclusion: Overall, erenumab treatment significantly reduced the burden of migraine, leading to improved quality of life for patients and their families over a treatment period of 2 years.
Keywords: Headache Impact Test‐6; Impact of Migraine on Partners and Adolescent Children; chronic and episodic migraine; drug holiday; medication overuse; monthly migraine days.
© 2025 The Author(s). Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016 [published correction appears in Lancet. 2017;390(10106):e38]. Lancet. 2017;390(10100):1211‐1259. doi: 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

