Global patterns of utilization of noninvasive tests for the clinical management of metabolic dysfunction-associated steatotic liver disease
- PMID: 40304566
- PMCID: PMC12045536
- DOI: 10.1097/HC9.0000000000000678
Global patterns of utilization of noninvasive tests for the clinical management of metabolic dysfunction-associated steatotic liver disease
Abstract
Background: Noninvasive tests (NITs) are used to risk-stratify metabolic dysfunction-associated steatotic liver disease. The aim was to survey global patterns of real-world use of NITs.
Methods: A 38-item survey was designed by the Global NASH Council. Providers were asked about risks for advanced fibrosis, which NITs (cutoff values) they use to risk-stratify liver disease, monitor progression, and which professional guidelines they follow.
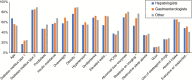
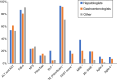
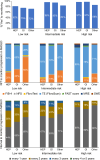
Results: A total of 321 participants from 43 countries completed the survey (54% hepatologists, 28% gastroenterologists, and 18% other). Of the respondents, 85% would risk-stratify patients with type 2 diabetes, obesity (82%), or abnormal liver enzymes (73%). Among NITs to rule out significant or advanced fibrosis, transient elastography (TE) and fibrosis-4 (FIB-4) were most used, followed by NAFLD Fibrosis Score, Enhanced Liver Fibrosis, and magnetic resonance elastography. The cutoffs for ruling out significant fibrosis varied considerably between practices and from guidelines, with only 50% using TE <8 kPa, 65% using FIB-4 <1.30 for age <65, and 41% using FIB-4 <2.00 for age ≥65. Similar variability was found for ruling in advanced fibrosis, where thresholds of FIB-4 ≥2.67 and TE ≥10 kPa were used by 20% and 17%, respectively. To establish advanced fibrosis, 48% would use 2 NITs while 23% would consider 1 NIT, and 17% would confirm with liver biopsy. TE was used by >75% to monitor, and 66% would monitor (intermediate or high risk) annually. Finally, 65% follow professional guideline recommendations regarding NITs.
Conclusions: In clinical practice, there is variability in NIT use and their thresholds. Additionally, there is suboptimal adherence to professional societies' guidelines.
Keywords: 2D-SWE; ELF; Fib-4; VCTE; risk stratification.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Zobair M. Younossi is a consultant or has received research funding from Intercept, Cymabay, Boehringer Ingelheim, Ipsen, Gilead Sciences, Inventiva, BMS, GSK, Novo Nordisk, Siemens, Madrigal, Merck, Akero, and Abbott.
Figures
References
-
- Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. . NAFLD Consensus Consortium. Advancing the global public health agenda for NAFLD: A consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60–78. - PubMed
-
- Lee HH, Lee HA, Kim EJ, Kim HY, Kim HC, Ahn SH, et al. . Metabolic dysfunction–associated steatotic liver disease and risk of cardiovascular disease. Gut. 2024;73:533–540. - PubMed
-
- Roderburg C, Kostev K, Mertens A, Luedde T, Loosen SH. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of extrahepatic cancer. Gut. 2023;72:2383–2384. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

