Safety Monitoring of Omaveloxolone in Friedreich Ataxia: Results from One Year of Clinical Treatment
- PMID: 40304846
- PMCID: PMC12089633
- DOI: 10.1007/s40120-025-00749-3
Safety Monitoring of Omaveloxolone in Friedreich Ataxia: Results from One Year of Clinical Treatment
Abstract
Introduction: Omaveloxolone, the only approved medication for Friedreich ataxia (FRDA), is an NRF2 activator available since July 2023. We examined safety monitoring of omaveloxolone administration over the first 12 months of administration.
Methods: We recorded baseline and follow-up serum transaminase, albumin, total bilirubin, cholesterol, and brain natriuretic peptide (BNP) values as well as adverse events over 1 year in patients initiating commercial omaveloxolone therapy.
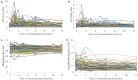
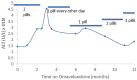
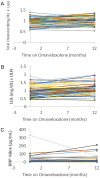
Results: Access to omaveloxolone was obtained in 236 of individuals for whom it was prescribed. Side effects were noted in 23.8% of patient with the most common being gastrointestinal upset, headache, and fatigue baseline. Twenty-one patients (8.9%) permanently discontinued the drug during the first year. Over the first year, 56.6% of patients had at least one transaminase value above the upper limit of normal at some point. Elevations largely occurred over the first 3 months of therapy, and after 6 months of dosing, only 8.6% of patients had elevations in transaminases. Elevations were generally < 3 × the upper limit of normal and decreased with temporary pausing of the drug or dose reduction. Few changes were noted in albumin or bilirubin, and such changes did not parallel changes in transaminases, suggesting they are independent events. BNP values were generally unchanged throughout the year, and no systematic changes in blood counts were noted. Cholesterol and low-density lipoprotein (LDL) elevations were mild.
Conclusions: Most patients with FRDA eventually had access to omaveloxolone, and it was generally well tolerated. Side effects were modest, and, overall, most patients remained on the drug. Abnormalities in serum liver function tests were limited to transaminases, resolved with dose pausing or reduction, and diminished markedly over time. Thus, the safety features of omaveloxolone after administration largely resemble the favorable features noted during clinical trials.
Keywords: Brain natriuretic peptide; Mitochondria transaminase; Movement disorder; Omaveloxolone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: David Lynch receives grants from Reata/Biogen pharmaceuticals for performance of the MOXIE and planned studies of omaveloxolone. He also receives grants from Larimar, PTC, Neurocrine, Solid Biosciences, the NIH, FDA, FARA and MDA. Katherine Gunther, Victoria Profeta, Medina Keita, Courtney Park, McKenzie Wells, Sonal Sharma, and Kimberly Schadt have nothing to disclose. Ethical Approval: The study was approved through the CHOP IRB as study 2609 and performed in accordance with the Helsinki Declaration of 1964. All subjects provided informed consent to participate in the study.
Figures
References
-
- Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589–620. - PubMed
-
- Pandolfo M. Friedreich ataxia. Arch Neurol. 2008;65:1296–303. - PubMed
-
- Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335:1169–75. - PubMed
-
- Tsou AY, Paulsen EK, Lagedrost SJ, Perlman SL, Mathews KD, Wilmot GR, et al. Mortality in Friedreich ataxia. J Neurol Sci. 2011;307:46–9. - PubMed
LinkOut - more resources
Full Text Sources

