Navigating antibody‒drug conjugates (ADCs): from metastatic to early breast cancer treatment strategies
- PMID: 40304863
- PMCID: PMC12310797
- DOI: 10.1007/s10637-025-01525-8
Navigating antibody‒drug conjugates (ADCs): from metastatic to early breast cancer treatment strategies
Abstract
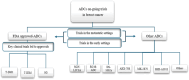
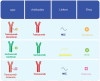
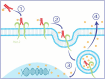
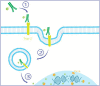
We are currently living in the era of precision medicine, and antibody‒drug conjugates (ADCs) represent promising advancements in targeted cancer therapy. While several ADCs have been investigated over the years, only three have gained FDA approval for breast cancer (BC): ado-trastuzumab emtansine (T-DM1/Kadcyla), trastuzumab deruxtecan (T-DXd/Enhertu), and sacituzumab govitecan (SG/Trodelvy). This review focuses on the three approved ADCs for BC, reviewing the trials that led to their approval and detailing the ongoing trials testing their clinical efficacy and safety profiles. We examine ongoing trials targeting both metastatic and early-stage patients. Notably, we explore trials incorporating investigational ADCs into early management strategies, addressing the unique challenges of biomarker identification, target toxicity, and cost-effectiveness. By summarizing the current landscape of FDA-approved and investigational ADCs, this study highlights the evolving nature of BC treatment. Preliminary findings from ongoing trials suggest that early integration of ADCs can lead to significant improvements in disease-free survival, reinforcing their role in personalized medicine. As research advances, ADCs are likely to become a cornerstone of breast cancer treatment, providing new hope for better patient outcomes.
Keywords: ADCs; Antibody‒drug conjugates; Early breast cancer; Metastatic breast cancer; Trials.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and informed consent: Not applicable. Competing interests: The authors declare no competing interests.
Figures
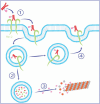

References
-
- Research C for DE and. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. FDA [Internet]. 2019 Dec 20 [cited 2024 Oct 5]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro...
-
- Research C for DE and. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA [Internet]. 2024 Aug 9 [cited 2024 Oct 5]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grant...
-
- Cancer Network [Internet]. 2013 [cited 2024 Oct 5]. FDA approves T-DM1 (Kadcyla) for HER2-positive breast cancer. Available from: https://www.cancernetwork.com/view/fda-approves-t-dm1-kadcyla-her2-posit...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

