Timing of Complementary Feeding in Preterm Infants and Prevalence of Overweight and Obesity: A Randomized Clinical Trial
- PMID: 40305025
- PMCID: PMC12044495
- DOI: 10.1001/jamanetworkopen.2025.2968
Timing of Complementary Feeding in Preterm Infants and Prevalence of Overweight and Obesity: A Randomized Clinical Trial
Abstract
Importance: The appropriate time for initiating complementary feeding in preterm infants is crucial for optimizing growth and preventing long-term health issues, such as overweight. Currently, there are no established guidelines for preterm infants.
Objective: To investigate the effect of initiating complementary feeding at corrected age 12 weeks vs 17 weeks on the prevalence of overweight and obesity at corrected age 2 years in preterm infants.
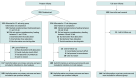
Design, setting, and participants: This multicenter randomized clinical trial was conducted between May 13, 2016, and April 26, 2021, with follow-up completed in December 2023. Seventeen hospitals in the Netherlands recruited preterm infants born between gestational age (GA) 30 and 36 weeks, supplemented with a reference group of full-term infants.
Intervention: Preterm infants were randomized to initiating complementary feeding at corrected age 12 weeks (early group) or 17 weeks (late group).
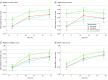
Main outcomes and measures: The primary outcome was the prevalence of overweight and obesity at corrected age 2 years measured using logistic mixed models. Secondary outcomes were height, weight, head circumference, body mass index, and z scores at corrected age 1 and 2 years, as well as neurodevelopment, atopic dermatitis score, and health-related quality of life.
Results: A total of 255 preterm infants were included and randomly assigned, with 131 (51.4%; median [IQR] GA, 34 weeks 2 days [32 weeks 5 days to 35 weeks 1 day]; 77 male [58.8%]) allocated to the early group and 124 (48.6%; median [IQR] GA, 34 weeks 0 days [32 weeks 6 days to 34 weeks 6 days]; 62 male [50.0%]) allocated to the late group. A total of 159 full-term infants (median [IQR] GA, 40 weeks 0 days [39 week 0 days to 41 weeks 0 days]; 84 female [52.8%]) were included as the reference group. Information on the primary outcome was available for 226 preterm infants (88.6%) and 144 full-term infants (90.6%). At corrected age 2 years, the prevalence of overweight was 6.0% (95% CI, 2.7%-11.5%) in the early group and 5.5% (95% CI, 2.3%-11.1%) in the late group. For obesity, the prevalence was 1.7% (95% CI, 0.3%-5.5%) in the early group and 1.8% (95% CI, 0.3%-5.9%) in the late group. The full-term reference group showed a higher prevalence of overweight (9.0%; 95% CI, 5.1%-14.6%) and obesity (3.5%; 95% CI, 1.3%-7.5%).
Conclusions and relevance: In this randomized clinical trial of preterm infants, initiating complementary feeding between corrected age 12 and 17 weeks did not affect overweight and obesity prevalence at corrected age 2 years.
Trial registration: Onderzoekmetmensen.nl Identifier: NL-OMON53076.
Conflict of interest statement
Figures
References
-
- World Health Organization . Global strategy for infant and young child feeding. December 22, 2003. Accessed March 5, 2024. https://www.who.int/publications/i/item/9241562218
-
- World Health Organization . Infant and young child feeding. December 20, 2023. Accessed March 5, 2024. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-...
-
- Agostoni C, Decsi T, Fewtrell M, et al. ; ESPGHAN Committee on Nutrition . Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46(1):99-110. doi:10.1097/01.mpg.0000304464.60788.bd - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous