Comparative Insights on IL-5 Targeting with Mepolizumab and Benralizumab: Enhancing EGPA Treatment Strategies
- PMID: 40305320
- PMCID: PMC12025051
- DOI: 10.3390/biom15040544
Comparative Insights on IL-5 Targeting with Mepolizumab and Benralizumab: Enhancing EGPA Treatment Strategies
Abstract
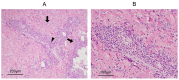
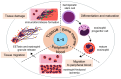
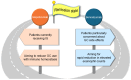
Eosinophilic granulomatosis with polyangiitis (EGPA) is a necrotizing vasculitis characterized by extravascular granulomas and eosinophilia in both blood and tissues. Eosinophils, which play a critical role in the pathophysiology of EGPA, require interleukin (IL)-5 for maturation in the bone marrow and migration to tissues. Glucocorticoids and immunosuppressants have been the cornerstone of treatment; however, their side effects have imposed a significant burden on many patients. Mepolizumab, an antibody that binds to and neutralizes IL-5, demonstrated efficacy in controlling disease activity in EGPA in the MIRRA trial conducted in 2017. In 2024, benralizumab, an IL-5 receptor alpha antagonist, was shown to be non-inferior to mepolizumab in efficacy against EGPA in the MANDARA trial. Both drugs were originally used for severe asthma and have benefited EGPA by reducing eosinophil counts. Due to differences in pharmacological structure and pharmacokinetics, the degree of eosinophil suppression varies between the two agents, and recent studies suggest that they may also affect inflammatory and homeostatic eosinophils differently. This review summarizes the latest insights into the pathophysiology of EGPA, highlights the similarities and differences between the two drugs, and discusses future treatment strategies for EGPA based on current clinical unmet needs, including drug selection.
Keywords: benralizumab; eosinophil; eosinophilic granulomatosis with polyangiitis; interleukin-5; mepolizumab.
Conflict of interest statement
R.W. received a research grant from AbbVie and speaker’s fee from AbbVie, Asahi Kasei, Chugai, Eli Lilly, GSK, and UCB Japan. MH received research grants and/or speaker’s fee from AbbVie, Asahi Kasei, Astellas, Bristol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, Taisho Toyama, Tanabe Mitsubishi, Towa Pharma and UCB Japan. These pharmaceutical companies had no role in the writing of the manuscript.
Figures

References
-
- Sorin B., Papo M., Sinico R.A., Teixeira V.S., Venhoff N., Urban M.-L., Iudici M., Mahrhold J., Locatelli F., Cassone G., et al. Glucocorticoids versus glucocorticoids plus cyclophosphamide in eosinophilic granulomatosis with polyangiitis with poor-prognosis factors. J. Autoimmun. 2024;149:103338. doi: 10.1016/j.jaut.2024.103338. - DOI - PubMed
-
- Hellmich B., Sanchez-Alamo B., Schirmer J.H., Berti A., Blockmans D., Cid M.C., Holle J.U., Hollinger N., Karadag O., Kronbichler A., et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024;83:30–47. doi: 10.1136/ard-2022-223764. - DOI - PubMed
-
- Emmi G., Bettiol A., Gelain E., Bajema I.M., Berti A., Burns S., Cid M.C., Tervaert J.W.C., Cottin V., Durante E., et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023;19:378–393. doi: 10.1038/s41584-023-00958-w. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

