Molecule-Environment Embedding with Quantum Monte Carlo: Electrons Interacting with Drude Oscillators
- PMID: 40305468
- PMCID: PMC12080123
- DOI: 10.1021/acs.jctc.5c00108
Molecule-Environment Embedding with Quantum Monte Carlo: Electrons Interacting with Drude Oscillators
Abstract
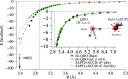
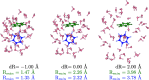
We present a comprehensive investigation of the El-QDO embedding method [Phys. Rev. Lett. 131, 228001 (2023)], where molecular systems described through the electronic Hamiltonian are immersed in a bath of charged quantum harmonic oscillators, i.e., quantum Drude oscillators (QDOs). In the El-QDO model, the entire system of electrons and drudons─the quantum particles in the QDOs─is modeled through a single Hamiltonian which is solved through quantum Monte Carlo (QMC) methods. We first describe the details of the El-QDO Hamiltonian, of the proposed El-QDO ansatz, and of the QMC algorithms implemented to integrate both electronic and drudonic degrees of freedom. Then we analyze short-range regularization functions for the interacting potential between electrons and QDOs in order to accurately treat equilibrium and repulsive regions, resolving the overpolarization error that occurs between the electronic system and the environment. After benchmarking various regularization (damping) functions on the cases of argon and water dimers, the El-QDO method is applied to study the solvation energies of the benzene and water dimers, verifying the accuracy of the El-QDO approach compared to accurate fully electronic ab initio calculations. Furthermore, through the comparison of the El-QDO interaction energies with the components of Symmetry-Adapted Perturbation Theory calculations, we illustrate the El-QDO's explicit many-body treatment of electrostatic, polarization, and dispersion interactions between the electronic subsystem and the environment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Molecules in Environments: Toward Systematic Quantum Embedding of Electrons and Drude Oscillators.Phys Rev Lett. 2023 Dec 1;131(22):228001. doi: 10.1103/PhysRevLett.131.228001. Phys Rev Lett. 2023. PMID: 38101380
-
Quantum Drude oscillators coupled with Coulomb potential as an efficient model for bonded and non-covalent interactions in atomic dimers.J Chem Phys. 2024 Mar 7;160(9):094309. doi: 10.1063/5.0196690. J Chem Phys. 2024. PMID: 38445736
-
Universal Pairwise Interatomic van der Waals Potentials Based on Quantum Drude Oscillators.J Chem Theory Comput. 2023 Nov 14;19(21):7895-7907. doi: 10.1021/acs.jctc.3c00797. Epub 2023 Oct 24. J Chem Theory Comput. 2023. PMID: 37875419 Free PMC article.
-
Optimized Quantum Drude Oscillators for Atomic and Molecular Response Properties.J Phys Chem Lett. 2023 Jul 13;14(27):6217-6223. doi: 10.1021/acs.jpclett.3c01221. Epub 2023 Jun 29. J Phys Chem Lett. 2023. PMID: 37385598 Free PMC article.
-
Modeling coarse-grained van der Waals interactions using dipole-coupled anisotropic quantum Drude oscillators.J Comput Chem. 2023 May 5;44(12):1164-1173. doi: 10.1002/jcc.27073. Epub 2023 Jan 16. J Comput Chem. 2023. PMID: 36645104
References
-
- Khare R.; Mielke S. L.; Paci J. T.; Zhang S.; Ballarini R.; Schatz G. C.; Belytschko T. Coupled quantum mechanical/molecular mechanical modeling of the fracture of defective carbon nanotubes and graphene sheets. Phys. Rev. B 2007, 75, 07541210.1103/PhysRevB.75.075412. - DOI
LinkOut - more resources
Full Text Sources
