Heuristic energy-based cyclic peptide design
- PMID: 40305587
- PMCID: PMC12043242
- DOI: 10.1371/journal.pcbi.1012290
Heuristic energy-based cyclic peptide design
Abstract
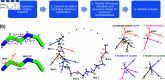
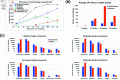
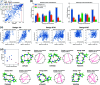
Rational computational design is crucial to the pursuit of novel drugs and therapeutic agents. Meso-scale cyclic peptides, which consist of 7-40 amino acid residues, are of particular interest due to their conformational rigidity, binding specificity, degradation resistance, and potential cell permeability. Because there are few natural cyclic peptides, de novo design involving non-canonical amino acids is a potentially useful goal. Here, we develop an efficient pipeline (CyclicChamp) for cyclic peptide design. After converting the cyclic constraint into an error function, we employ a variant of simulated annealing to search for low-energy peptide backbones while maintaining peptide closure. Compared to the previous random sampling approach, which was capable of sampling conformations of cyclic peptides of up to 14 residues, our method both greatly accelerates the computation speed for sampling conformations of small macrocycles (ca. 7 residues), and addresses the high-dimensionality challenge that large macrocycle designs often encounter. As a result, CyclicChamp makes conformational sampling tractable for 15- to 24-residue cyclic peptides, thus permitting the design of macrocycles in this size range. Microsecond-length molecular dynamics simulations on the resulting 15, 20, and 24 amino acid cyclic designs identify designs with kinetic stability. To test their thermodynamic stability, we perform additional replica exchange molecular dynamics simulations and generate free energy surfaces. Three 15-residue designs, one 20-residue and one 24-residue design emerge as promising candidates.
Copyright: © 2025 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Update of
-
Heuristic energy-based cyclic peptide design.bioRxiv [Preprint]. 2025 Feb 28:2024.07.03.601955. doi: 10.1101/2024.07.03.601955. bioRxiv. 2025. Update in: PLoS Comput Biol. 2025 Apr 30;21(4):e1012290. doi: 10.1371/journal.pcbi.1012290. PMID: 39005429 Free PMC article. Updated. Preprint.
Similar articles
-
Heuristic energy-based cyclic peptide design.bioRxiv [Preprint]. 2025 Feb 28:2024.07.03.601955. doi: 10.1101/2024.07.03.601955. bioRxiv. 2025. Update in: PLoS Comput Biol. 2025 Apr 30;21(4):e1012290. doi: 10.1371/journal.pcbi.1012290. PMID: 39005429 Free PMC article. Updated. Preprint.
-
De Novo Design of Cyclic Peptide Binders Based on Fragment Docking and Assembling.J Chem Inf Model. 2025 Apr 28;65(8):4206-4218. doi: 10.1021/acs.jcim.5c00088. Epub 2025 Apr 14. J Chem Inf Model. 2025. PMID: 40223692
-
Accurate Structure Prediction and Conformational Analysis of Cyclic Peptides with Residue-Specific Force Fields.J Phys Chem Lett. 2016 May 19;7(10):1805-10. doi: 10.1021/acs.jpclett.6b00452. Epub 2016 May 2. J Phys Chem Lett. 2016. PMID: 27128113
-
Elucidating Solution Structures of Cyclic Peptides Using Molecular Dynamics Simulations.Chem Rev. 2021 Feb 24;121(4):2292-2324. doi: 10.1021/acs.chemrev.0c01087. Epub 2021 Jan 11. Chem Rev. 2021. PMID: 33426882 Free PMC article. Review.
-
Computational Opportunities and Challenges in Finding Cyclic Peptide Modulators of Protein-Protein Interactions.Methods Mol Biol. 2019;2001:73-95. doi: 10.1007/978-1-4939-9504-2_5. Methods Mol Biol. 2019. PMID: 31134568 Review.
Cited by
-
Computational structure prediction of lanthipeptides with NMR data reveals underappreciated peptide flexibility.Protein Sci. 2025 Sep;34(9):e70252. doi: 10.1002/pro.70252. Protein Sci. 2025. PMID: 40823923 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

