Inhalable myofibroblast targeting nanoparticles for synergistic treatment of pulmonary fibrosis
- PMID: 40305619
- PMCID: PMC12042884
- DOI: 10.1126/sciadv.adv9571
Inhalable myofibroblast targeting nanoparticles for synergistic treatment of pulmonary fibrosis
Abstract
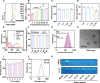
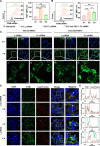
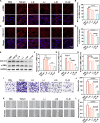
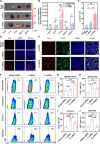
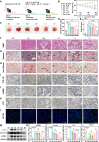
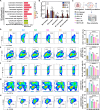
Pulmonary fibrosis (PF) is a life-threatening interstitial lung disease, characterized by excessive fibroblast activation and collagen deposition, leading to progressive pulmonary function decline and limited therapeutic efficacy. Here, the inhalable, myofibroblast-targeted, and pH-responsive liposomes (FL-NI) were developed for effective codelivery of nintedanib, a mainstream antifibrotic drug in clinic, and siIL11, a small interfering RNA that silences the key profibrosis cytokine IL-11. Notably, FL-NI achieved a 117.8% increase in pulmonary drug delivery by noninvasive inhalation and a 71.5% increase in delivery specifically to fibroblast activation protein-positive myofibroblasts while reducing nonspecific immune cell and epithelial uptake by 29.8 and 55.8%, respectively. The accurate inhalation codelivery of nintedanib and siIL11 into myofibroblasts achieved synergistic effects, effectively enhanced myofibroblast deactivation, reduced pathological collagen deposition by 50.8%, and promoted epithelial tissue repair. FL-NI remodeled the aberrant immune microenvironment without inducing systemic toxicities. Therefore, this work demonstrated the notable potential for this pluripotent strategy for improving PF outcomes and its promising clinical translation.
Figures


References
-
- Raghu G., Remy-Jardin M., Richeldi L., Thomson C. C., Inoue Y., Johkoh T., Kreuter M., Lynch D. A., Maher T. M., Martinez F. J., Molina-Molina M., Myers J. L., Nicholson A. G., Ryerson C. J., Strek M. E., Troy L. K., Wijsenbeek M., Mammen M. J., Hossain T., Bissell B. D., Herman D. D., Hon S. M., Kheir F., Khor Y. H., Macrea M., Antoniou K. M., Bouros D., Buendia-Roldan I., Caro F., Crestani B., Ho L., Morisset J., Olson A. L., Podolanczuk A., Poletti V., Selman M., Ewing T., Jones S., Knight S. L., Ghazipura M., Wilson K. C., Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Resp. Cri.t Care Med. 205, E18–E47 (2022). - PMC - PubMed
-
- Podolanczuk A. J., Thomson C. C., Remy-Jardin M., Richeldi L., Martinez F. J., Kolb M., Raghu G., Idiopathic pulmonary fibrosis: State of the art for 2023. Eur. Respir. J. 61, 2200957 (2023). - PubMed
-
- Bellou V., Belbasis L., Evangelou E., Tobacco smoking and risk for pulmonary fibrosis: A prospective cohort study from the UK Biobank. Chest 160, 983–993 (2021). - PubMed
-
- Borie R., Kannengiesser C., Antoniou K., Bonella F., Crestani B., Fabre A., Froidure A., Galvin L., Griese M., Grutters J. C., Molina-Molina M., Poletti V., Prasse A., Renzoni E., van der Smagt J., van Moorsel C. H. M., European Respiratory Society statement on familial pulmonary fibrosis. Eur. Respir. J. 61, 2201383 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

