Introduction of a phenylalanine sink in fast growing cyanobacterium Synechococcus elongatus PCC 11801 leads to improved PSII efficiency, linear electron transport, and carbon fixation
- PMID: 40305870
- PMCID: PMC12043352
- DOI: 10.1111/tpj.70129
Introduction of a phenylalanine sink in fast growing cyanobacterium Synechococcus elongatus PCC 11801 leads to improved PSII efficiency, linear electron transport, and carbon fixation
Abstract
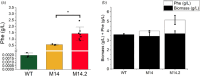
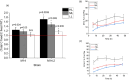
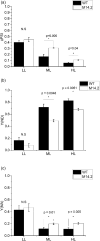
Cyanobacteria are investigated for fundamental photosynthesis research and sustainable production of valuable biochemicals. However, low product titer and biomass productivities are major bottlenecks to the economical scale-up. Recent studies have shown that the introduction of a metabolic sink, such as sucrose, 2,3-butanediol, and 2-phenyl ethanol, in cyanobacteria improves carbon fixation by relieving the "sink" limitation of photosynthesis. However, the impact of light intensity on the behavior of this sink-derived enhancement in carbon fixation is not well understood and is necessary for translation to outdoor cultivation. Here, using random mutagenesis, we engineered Synechococcus elongatus PCC 11801 to overproduce 1.24 g L-1 phenylalanine (Phe) in 3 days, identified L531W in the TolC protein as an important driver of Phe efflux, and investigated the effect of light intensity on total carbon fixation. We found that low light results in competition between biomass and Phe, whereas under excess light, a higher flux of fixed carbon is directed to the Phe sink. The introduction of the Phe sink improves the quantum yields of photosystem I and II with a concomitant increase in the total electron flow leading to nearly 70% increase in carbon fixation at high light in the mutant strain. Additionally, the cyclic electron flow decreased, which has implications for the ATP/NADPH production ratio. Our data highlight how light intensity affects the sink-derived enhancement in carbon fixation, the role of CEF to balance the source-sink demand for ATP and NADPH, and the enhancement of inorganic carbon fixation in cyanobacteria with an engineered sink.
Keywords: Synechococcus elongatus; carbon fixation; cyclic electron transport; linear electron transport; phenylalanine; photosynthetic efficiency; source‐sink relationship.
© 2025 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors report that strains M14 and M14.2, described in this work, are included in a US patent application 17/859837 filed by Purdue Research Foundation, Office of Technology Commercialization, with inventors listed as Arnav Deshpande and John A. Morgan.
Figures




Similar articles
-
Increased Photochemical Efficiency in Cyanobacteria via an Engineered Sucrose Sink.Plant Cell Physiol. 2016 Dec;57(12):2451-2460. doi: 10.1093/pcp/pcw169. Epub 2016 Oct 13. Plant Cell Physiol. 2016. PMID: 27742883
-
Adjustments to Photosystem Stoichiometry and Electron Transfer Proteins Are Key to the Remarkably Fast Growth of the Cyanobacterium Synechococcus elongatus UTEX 2973.mBio. 2018 Feb 6;9(1):e02327-17. doi: 10.1128/mBio.02327-17. mBio. 2018. PMID: 29437923 Free PMC article.
-
A carbon sink pathway increases carbon productivity in cyanobacteria.Metab Eng. 2015 May;29:106-112. doi: 10.1016/j.ymben.2015.03.006. Epub 2015 Mar 14. Metab Eng. 2015. PMID: 25777135
-
Interactions between CCM and N2 fixation in Trichodesmium.Photosynth Res. 2011 Sep;109(1-3):73-84. doi: 10.1007/s11120-010-9611-3. Epub 2010 Dec 29. Photosynth Res. 2011. PMID: 21190135 Review.
-
A survey of carbon fixation pathways through a quantitative lens.J Exp Bot. 2012 Mar;63(6):2325-42. doi: 10.1093/jxb/err417. Epub 2011 Dec 26. J Exp Bot. 2012. PMID: 22200662 Review.
References
-
- Abramson, B.W. , Kachel, B. , Kramer, D.M. & Ducat, D.C. (2016) Increased photochemical efficiency in cyanobacteria via an engineered sucrose sink. Plant & Cell Physiology, 57, 2451–2460. - PubMed
-
- Allen, J.F. (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends in Plant Science, 8, 15–19. - PubMed
-
- Baker, N.R. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89–113. - PubMed
-
- Bongaerts, J. , Krämer, M. , Müller, U. , Raeven, L. & Wubbolts, M. (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metabolic Engineering, 3, 289–300. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

