Obesity and its management in primary care setting
- PMID: 40305970
- PMCID: PMC12233002
- DOI: 10.1016/j.jdiacomp.2025.109045
Obesity and its management in primary care setting
Abstract
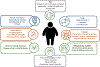
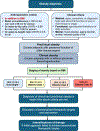
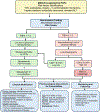
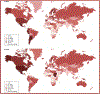
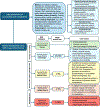
Obesity is a worldwide epidemic affecting adults and children, regardless of their socioeconomic status. Significant progress has been made in understanding the genetic causes contributing to obesity, shedding light on a portion of cases worldwide. In young children with severe obesity however, recessive mutations, i.e., leptin or leptin receptor deficiency should be sought. Much more has been learned about the far-reaching impact of obesity on complications, including cardiovascular disease, liver and kidney dysfunction, diabetes, inflammation, hypertension, sleep, cancer, and the eye. Preventive strategies, particularly in children, are crucial for reducing obesity rates and mitigating its long-term complications. While dietary modifications and lifestyle changes remain the cornerstone of obesity prevention or treatment, recent advancements have introduced highly effective pharmacological options complementing weight-reduction surgery. Newer medications, like incretin-based therapies including glucagon-like peptide-1 agonists (GLP-1RA), have demonstrated remarkable efficacy in promoting weight loss, offering new insights into margining obesity-related conditions. Primary care providers, whether treating adults or children, play a pivotal role in preventing obesity, initiating treatment, and making onward referrals to specialists to assist in managing obesity and obesity-related complications.
Keywords: Complications; Lifestyle; Metabolic surgery; Obesity; Pharmacotherapy; Primary care.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest MI has consultancy agreement with and received speaker honoraria from Novo-Nordisk, Boehringer Ingelheim, Medtronic and Pfizer; EB has received speaker honoraria from Eli Lilly, Novo-Nordisk, Sevier, Boehringer Ingelheim, Sanofi-Aventis, and Novartis; Abbott; JAA no conflicts of interest related to this manuscript: JB no conflicts of interest related to this manuscript; VB no conflict of interest to declare; AC (Israel) has received advisory board and/or honoraria from Novonordisk, Sanofi, Boehringher Ingelheim, AstraZeneca, Elli Lilly, Pfizer; AC (Italy) has received honoraria from AstraZeneca, Berlin Chemie, Merck, Novo Nordisk, and Roche Diagnostics. AC has received consulting fees from AstraZeneca, Bayer, Elsevier, Roche Diagnostics, Sevier, and Theras. AC has participated on advisory boards for Eli Lilly and Roche Diagnostics. This work was supported, in part, by the Italian Ministry of Health through Ricerca Corrente to IRCCS MultiMedica; FC Research Grants from King Gustav V and Queen Victoria Foundation, Swedish Research Council, Swedish Heart & Lung Foundation; Consulting fees from Astra Zeneca, MSD, Pfizer, Novo Nordisk; payment or honoraria from Astra Zeneca, Bristol Meyers Squibb, Merck Sharp & Dohme, Novo Nordisk, Boehringer Ingelheim, Pfizer, Berlin Chemie; MJD has acted as a consultant/advisor and speaker for Eli Lilly, Novo Nordisk and Sanofi, has attended advisory boards for Amgen, AstraZeneca, Biomea Fusion, Carmot/Roche, Sanofi, Zealand Pharma Regeneron and EktaH and as a speaker for AstraZeneca and Boehringer Ingelheim. She has received grants from AstraZeneca, Boehringer Ingelheim and Novo Nordisk; FDD no conflict of interest to declare; RHE Amgen, Arrowhead, Better Co., 89bio, Lexicon, Novo Nordisk, Precision BioSciences, The Healthy Aging Co., Tolmar, UpToDate, WW (Weight Watchers); ANF is a scientific consultant for Eli Lilly, Novo Nordisk, Gila Therapeutics, and Morphic Medical; JG no conflict of interest to declare; OH no conflict of interest to declare; SI no conflict of interest to declare; SK no conflict of interest to declare; RDL no conflict of interest to declare; IL received research funding (paid to institution) and/or product from NovoNordisk, Boehringer-Ingelheim, Dexcom, Roche, Pfizer, Lilly. IL received research related consulting fees (paid to institution) from NovoNordisk. IL received advisory/consulting fees and/or other support from: Abbvie, Altimmune, Alveus Therapeutics, Amgen, Antag Therapeutics, Astra Zeneca, Bayer, Betagenon AB, Bioio Inc., Biomea, Boehringer-Ingelheim, Carmot, Cytoki Pharma, Eli Lilly, Intercept, Janssen/J&J, Juvena, Keros Therapeutic, Inc., Mediflix, Merck, Metsera, Neurocrine, Novo Nordisk, Pharmaventures, Pfizer, Regeneron, Roche, Sanofi, Shionogi, Source Bio, Structure Therapeutics, TARGET RWE, TERNS Pharma, The Comm Group, Verdiva Bio, WebMD, and Zealand Pharma; SM no conflict of interest to declare; OM no conflict of interest to declare; SDP consulted for Abbott, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Jiangsu Hengrui Pharmaceuticals Co., Merck Sharpe & Dohme, Novartis Pharmaceutical Co., Novo Nordisk, Sanofi, received grant support from Astra Zeneca, Boehringer Ingelheim speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck Sharpe & Dohme, Novartis Pharmaceutical Co., Novo Nordisk, Sanofi; FP has done lectures for Berlin-Chemie.This work was supported, in part, by the Italian Ministry of Health through Ricerca Corrente to IRCCS MultiMedica; MR has given lectures, received honoraria and research support and participated in conferences, advisory boards and clinical trials sponsored by many pharmaceutical companies, including Amgen, Astra Zeneca, Biodexa, Boehringer Ingelheim, Kowa, Eli Lilly, Meda, Mylan, Merck Sharp & Dohme, Novo Nordisk, Novartis, Roche, Sanofi and Servier; RDR is employee of Sciarc GmbH; CWlR reports grants from the EU Innovative Medicine Initiative, Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He serves on advisory boards and speakers' panels of Novo Nordisk, Roche, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Gila, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, Keyron, AstraZeneca, Arrowhead Pharma, Amgen, and Rhythm Pharma. ClR is the Chair of the Irish Society for Nutrition and Metabolism. ClR provides obesity clinical care in the My Best Weight clinic and Beyond BMI clinic and is a co-owner of these clinics; OS reported payment or honorariums for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Abbott Diagnostics, Lilly Deutschland, Boehringer Ingelheim, Bayer, Mannkind, and Lifescan and is a founder and CEO of Sciarc GmbH; PMS; has received consultancy agreement and honorarium for lecture: Boehringer Ingelheim, Novartis, Astra Zeneca Menarini and Roche diagnostic; VKS; no conflict of interest to declare; ES reported personal fees from Oxford Diabetes Trials Unit, Bayer, Berlin Chemie, Boehringer Ingelheim, Merck Serono, Novartis, and Novo Nordisk; AT; no conflict of interest to declare; DT no conflict of interest to declare; PV (France) discloses the following potential conflicts of interest: lectures for Abbott, AstraZeneca, Bayer, Eli Lilly, Hikma Pharmaceuticals, Merck Sharp & Dohme, Novo Nordisk, Novartis, Pfizer, Sanofi, Servier, Stendo; research grants from Abbott, Bristol-Myers Squibb–AstraZeneca, GEP Santé, Novo Nordisk, Société Medical Respiratoire, Vivisol; participation in expert committees for AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Daiichi Sankyo, Sanofi, Servier; GEU GEU is partly supported by research grants from National Institutes of Health (NIH/NATS UL 3UL1TR002378-05S2) from the Clinical and Translational Science Award program, and from National Institutes of Health and National Center for Research Resources (NIH/NIDDK 2P30DK111024–06). GEU has received research support (to Emory University) from Bayer, Abbott and Dexcom.
Figures




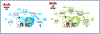

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

