Clinical effectiveness of a child-specific dynamic stretching programme, compared to usual care, for ambulant children with spastic cerebral palsy (SPELL trial): a parallel group randomized controlled trial
- PMID: 40306688
- PMCID: PMC12043370
- DOI: 10.1302/2633-1462.65.BJO-2024-0267
Clinical effectiveness of a child-specific dynamic stretching programme, compared to usual care, for ambulant children with spastic cerebral palsy (SPELL trial): a parallel group randomized controlled trial
Abstract
Aims: Dynamic muscle stretching exercises are one of the interventions frequently prescribed by physiotherapists for children with cerebral palsy (CP). However, there is wide variability in the exercise regimes used and limited evidence of their effectiveness. The SPELL trial will assess the clinical effectiveness of an individually tailored dynamic stretching programme, compared to usual care for ambulant children with spastic CP.
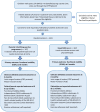
Methods: We are conducting a multicentre, two-arm, parallel group, superiority randomized controlled trial. We will recruit children aged four to 11 years with a diagnosis of spastic CP (bilateral or unilateral) and Gross Motor Function Classification System (GMFCS) levels I to III who are able to comply with assessment procedures and exercise programme with or without support. Participants will be recruited from at least 12 UK NHS Trust physiotherapy and related services. Participants (n = 334) will be randomized (centralized computer-generated one:one allocation ratio) to either: 1) a dynamic stretching exercise programme, with six one-to-one physiotherapy sessions over 16 weeks; or 2) usual NHS care, with a single physiotherapy session and an assessment, and advice regarding self-management and exercise.
Conclusion: The primary outcome is functional mobility measured using the child-/parent-reported Gait Outcomes Assessment List (GOAL) at six months. Secondary outcomes are: joint range of motion (Cerebral Palsy Integrated Pathway protocol) and motor function (timed up and go test) at six months; functional mobility (GOAL) at 12 months; independence (GOAL subdomain A); balance (GOAL subdomain A, B, D); pain and discomfort (GOAL subdomain C); health-related quality of life (youth version of the EuroQol five-dimension questionnaire (EQ-5D-Y)); educational attendance; exercise adherence; and additional physiotherapy treatment at six and 12 months. The primary analysis will be intention to treat.
© 2025 Theologis et al.
Conflict of interest statement
M. Andrew, G. B. Firth, V. Gregory Osborne, H. Gregory Osborne, L. Katchburian, D. J. Keene, J.R. Parr, D. C. Perry, R. Rapson, I. Rombach, and J. Ryan are co-applicants on the grant awarded by the National Institute of Health Research (NIHR) Health Technology Assessment (NIHR135131). G. B. Firth reports that as a co-applicant, a small amount of the funding for this study went towards his research department at Maidstone and Tunbridge Wells Trust. D. J. Keene is supported by the NIHR Exeter Biomedical Research Centre. J. Parr reports that his Newcastle University salary was funded by the NIHR in order to work on this study, and being Chair of the British Academy of Childhood Disability Strategic Research Group, whose Priority Setting led to the NIHR funding call for this study. D. C. Perry discloses NIHR Research Professorship funding from the NIHR Academy (NIHR301655).
Figures
Similar articles
-
Clinical effectiveness of an individually tailored strengthening programme, including progressive resistance exercises and advice, compared to usual care for ambulant adolescents with spastic cerebral palsy (ROBUST trial): a parallel group randomized controlled trial.Bone Jt Open. 2025 May 1;6(5):517-527. doi: 10.1302/2633-1462.65.BJO-2024-0268. Bone Jt Open. 2025. PMID: 40306695 Free PMC article.
-
Erratum.Mult Scler. 2016 Oct;22(12):NP9-NP11. doi: 10.1177/1352458515585718. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041800
-
Preschool HABIT-ILE: study protocol for a randomised controlled trial to determine efficacy of intensive rehabilitation compared with usual care to improve motor skills of children, aged 2-5 years, with bilateral cerebral palsy.BMJ Open. 2021 Mar 2;11(3):e041542. doi: 10.1136/bmjopen-2020-041542. BMJ Open. 2021. PMID: 33653745 Free PMC article.
-
Bisphosphonate use in children with cerebral palsy.Cochrane Database Syst Rev. 2021 Jul 5;7(7):CD012756. doi: 10.1002/14651858.CD012756.pub2. Cochrane Database Syst Rev. 2021. PMID: 34224134 Free PMC article.
-
Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review.Disabil Rehabil. 2019 May;41(10):1131-1151. doi: 10.1080/09638288.2017.1422035. Epub 2018 Jan 5. Disabil Rehabil. 2019. PMID: 29303007
Cited by
-
Clinical effectiveness of an individually tailored strengthening programme, including progressive resistance exercises and advice, compared to usual care for ambulant adolescents with spastic cerebral palsy (ROBUST trial): a parallel group randomized controlled trial.Bone Jt Open. 2025 May 1;6(5):517-527. doi: 10.1302/2633-1462.65.BJO-2024-0268. Bone Jt Open. 2025. PMID: 40306695 Free PMC article.
References
-
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. - PubMed
-
- No authors listed . SCOPE; [10 April 2025]. Cerebral palsy: introduction 2020.https://www.scope.org.uk/advice-and-support/cerebral-palsy-introduction date last. accessed.
LinkOut - more resources
Full Text Sources
Miscellaneous