Evaluation of Orientation-Dependent Cation-π Pairwise Effects within Collagen Triple Helices
- PMID: 40307192
- PMCID: PMC12086834
- DOI: 10.1021/acs.jpcb.4c08691
Evaluation of Orientation-Dependent Cation-π Pairwise Effects within Collagen Triple Helices
Abstract
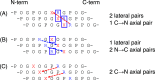
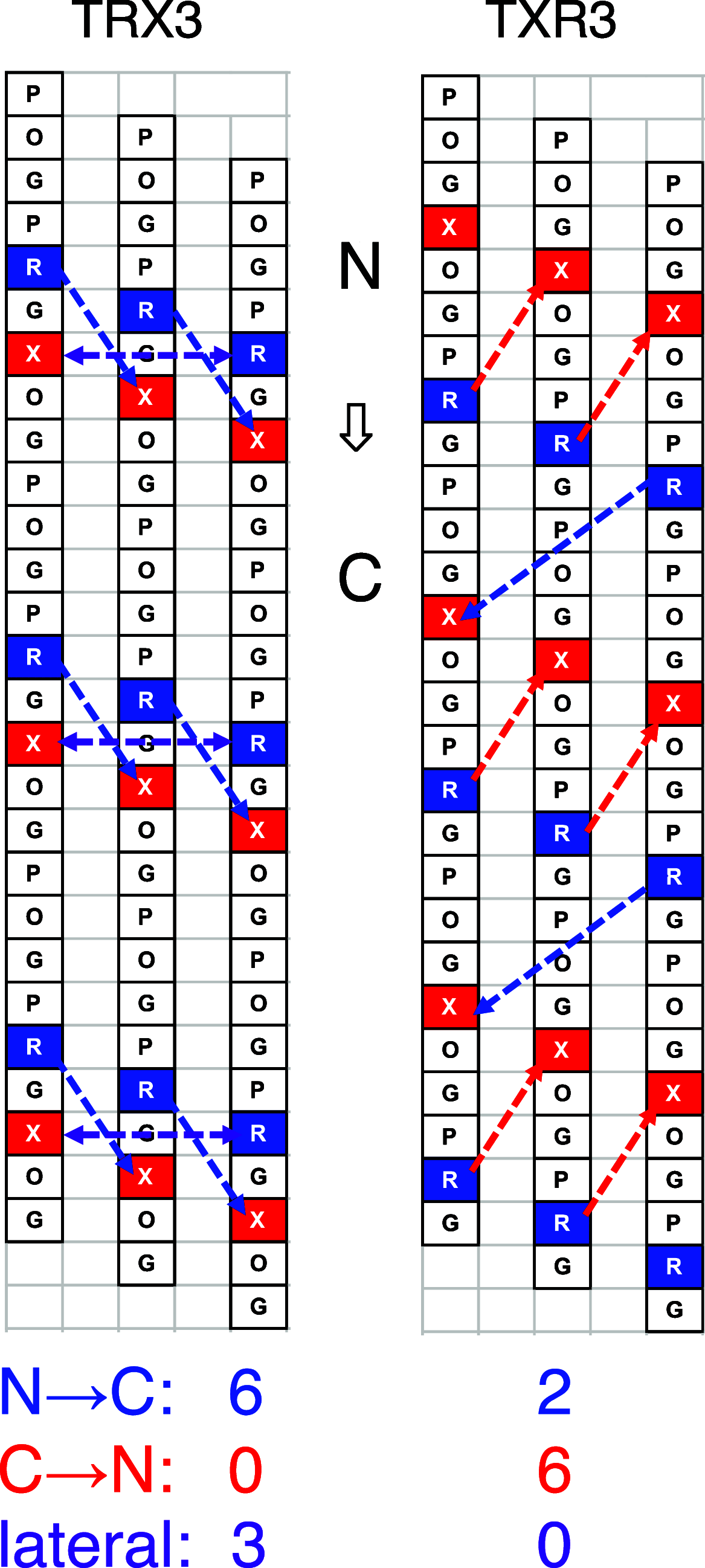
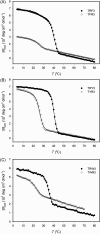
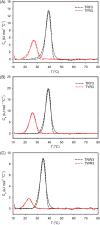
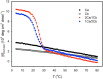
Various noncovalent interactions have been introduced to explore their impacts in folding a collagen triple helix. Among these interactions, the cation-π interaction represents one of the compelling forces stabilizing the triple helix. Still, the effects depend on the pairwise components and the orientation between the cationic and aromatic moieties. To gain more insights into this interaction within a collagen trimer, we prepared a series of collagen-mimetic peptides (CMPs) with cationic residues and aromatic residues incorporated to examine the contributions of two types of axial cation-π pairs (N → C and C → N cationic-to-aromatic pairwise) and the lateral cation-π pair. Circular dichroism (CD) measurements indicate that the N → C axial pairs have a significant stabilization effect. In contrast, the lateral and the C → N axial pairs destabilize the fold, and the lateral pairs cause the most destabilization consequences. We further designed and prepared the CMPs containing various lateral and axial cation-π pairs to investigate the coupling consequences in homotrimers and heterotrimers. From CD data, we found that the predicted differences in melting temperatures using individual cation-π pairwise contributions were comparable to the observed values for the designed homotrimers. CD and NMR measurements showed favorable cation-π interactions could effectively induce the folding of heterotrimers, in which the CMPs with more N → C axial pairs formed a more stable trimer than those containing a smaller number of N → C axial pairs. In this study, we have disclosed more valuable information about the properties of cation-π pairwise effects within a collagen triple helix, which can be considered in designing collagen-related peptides and materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Curtis R. W.; Chmielewski J. A comparison of the collagen triple helix and coiled-coil peptide building blocks on metal ion-mediated supramolecular assembly. Pept. Sci. 2021, 113 (2), e24190 10.1002/pep2.24190. - DOI
-
- Engel J.; Bachinger H. P.. Structure, Stability and Folding of the Collagen Triple Helix. In Collagen, Topics in Current Chemistry; Springer, 2005; Vol. 247, pp 7–33.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

