One-pot three-component synthesis of azaspirononatriene derivatives
- PMID: 40307286
- PMCID: PMC12043965
- DOI: 10.1038/s41598-025-97860-0
One-pot three-component synthesis of azaspirononatriene derivatives
Abstract
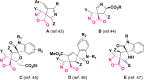
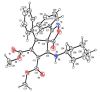
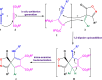
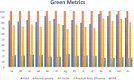
The present investigation has introduced a new class of isocyanide/acetylene-based multicomponent reactions (IAMCRs). These are a robust technique for efficiently synthesizing intricate spiro architectures through a zwitterionic adduct. The coupling reaction between the "in situ" generated dipoles of the isocyanide-acetylenic ester adducts and 3-alkyl-4-arylidene-isoxazol-5(4H)-one derivative presents a highly effective synthetic pathway for obtaining novel 1-oxo-2-oxa-3-azaspiro[4.4]nona-3,6,8-triene heterocycles. The broad range of substrates, standard experimental conditions, straightforward procedure, and impressive yields make our catalyst-free three-component approach highly practical and green, as it remarkably offers step-, time- and cost-effectiveness based on the green metrics.
Keywords: 1-Oxo-2-oxa-3-azaspiro[4.4]nona-3,6,8-triene; 4-Arylidene-isoxazol-5(4H)-one; Based multicomponent reaction; Isocyanide; Spiro heterocycle; Three-component reaction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: This article does not contain any studies with human participants or animals performed by the authors.
Figures
References
-
- Huang, J.-C. et al. Enantiomeric pairs of meroterpenoids with 11/5/6 spiro-heterocyclic systems from Hypericum kouytchense. Org. Chem. Front.9, 6475–6483 (2022). - DOI
-
- Mamaghani, M. & Hossein Nia, R. Recent developments in the MCRs synthesis of pyridopyrimidines and spiro-pyridopyrimidines. J. Heterocycl. Chem.54, 1700–1722 (2017). - DOI
-
- Khanna, P., Khanna, L., Thomas, S. J., Asiri, A. M. & Panda, S. S. Microwave assisted synthesis of spiro heterocyclic systems: A review. Curr. Org. Chem.22, 67–84 (2018). - DOI
LinkOut - more resources
Full Text Sources

