Cardioprotection by poloxamer 188 is mediated through increased endothelial nitric oxide production
- PMID: 40307302
- PMCID: PMC12043958
- DOI: 10.1038/s41598-025-97079-z
Cardioprotection by poloxamer 188 is mediated through increased endothelial nitric oxide production
Abstract
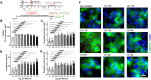
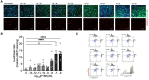
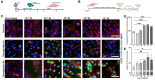
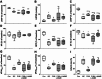
Ischemia/reperfusion (I/R) injury significantly contributes to the morbidity and mortality associated with cardiac events. Poloxamer 188 (P188), a non-ionic triblock copolymer, has been proposed to mitigate I/R injury by stabilizing cell membranes. However, the underlying mechanisms remain incompletely understood, particularly concerning endothelial cell (EC) function and nitric oxide (NO) production. We employed human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (CMs) and ECs to elucidate the effects of P188 on cellular survival, function, and NO secretion under simulated I/R conditions. iPSC-CMs contractility and iPSC-ECs' NO production were assessed following exposure to P188. Further, an isolated heart model using Brown Norway rats subjected to I/R injury was utilized to evaluate the ex-vivo cardioprotective effects of P188, examining cardiac function and NO production, with and without the administration of a NO inhibitor. In iPSC-derived models, P188 significantly preserved CM contractile function and enhanced cell viability after hypoxia/reoxygenation. Remarkably, P188 treatment led to a pronounced increase in NO secretion in iPSC-ECs, a novel finding demonstrating endothelial protective effects beyond membrane stabilization. In the rat isolated heart model, administration of P188 during reperfusion notably improved cardiac function and reduced I/R injury markers. This cardioprotective effect was abrogated by NO inhibition, underscoring the pivotal role of NO. Additionally, a dose-dependent increase in NO production was observed in non-ischemic rat hearts treated with P188, further establishing the critical function of NO in P188 induced cardioprotection. In conclusion, our comprehensive study unveils a novel role of NO in mediating the protective effects of P188 against I/R injury. This mechanism is evident in both cellular models and intact rat hearts, highlighting the potential of P188 as a therapeutic agent against I/R injury. Our findings pave the way for further investigation into P188's therapeutic mechanisms and its potential application in clinical settings to mitigate I/R-related cardiac dysfunction.
Keywords: Cardiac arrest; Copolymer; Ischemia/reperfusion injury; Myocardial infarction; NO; P188.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: All human cell protocols for this study were approved by the Stanford University Human Subjects Research Institutional Review Board (IRB). ARRIVE guidelines: All data in this study are reported in accordance with ARRIVE guidelines ( https://arriveguidelines.org ).
Figures
Update of
-
Cardioprotection by Poloxamer 188 is Mediated through Increased Endothelial Nitric Oxide Production.bioRxiv [Preprint]. 2024 May 20:2024.05.18.593838. doi: 10.1101/2024.05.18.593838. bioRxiv. 2024. Update in: Sci Rep. 2025 Apr 30;15(1):15170. doi: 10.1038/s41598-025-97079-z. PMID: 38826479 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

