A targeted literature review on the impact of tailored interventions on patient outcomes in oncology
- PMID: 40307508
- PMCID: PMC12074991
- DOI: 10.1038/s41388-025-03424-x
A targeted literature review on the impact of tailored interventions on patient outcomes in oncology
Abstract
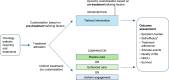
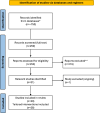
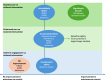
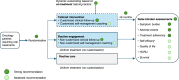
Non-pharmacological approaches to managing symptoms and side effects of cancer treatments are not always aligned with true needs and characteristics of patients. Without proactive patient-tailored interventions to anticipate, prevent, and manage side effects, risk of patients experiencing treatment interruption or discontinuation increases. A targeted literature review identified published studies evaluating the impact of tailored (customized based on individual patient characteristics) non-pharmacological interventions on patient outcomes in oncology versus routine care (usual clinical practice), enhanced care (support beyond routine care, including additional patient education, psychological support, and medication tracking), or uniform engagement (non-tailored support offered in a uniform manner). Thirty completed clinical studies were included. Approximately 50% of interventions across studies were remote health education/self-management programs, and the remaining provided clinical follow-up for symptom management. All types of tailored intervention led to positive patient outcomes versus routine care. Significant improvements were seen in favor of self-efficacy for self-management (patient's belief in their ability to manage own symptoms and treatment; p < 0.05) and symptom burden (overall as well as specific symptoms including anxiety, nausea, vomiting; all p < 0.05), although there were inconsistent effects on health-related quality of life (HRQoL), healthcare resource utilization (HCRU), and adherence. Compared with enhanced care, most studies showed no consistent improvement for tailored interventions in symptom burden, self-efficacy, HRQoL, HCRU or adherence, although benefits were slightly more common in larger/longer studies. Tailored interventions were not consistently better than uniform engagement in improving outcomes, although the number of studies was limited, with small sample sizes/short follow-ups. In summary, tailored interventions showed positive benefits versus routine or enhanced care (the latter only in larger and longer studies). No consistent benefit was observed compared with uniform engagement. Clinical outcomes were most sensitive to type of intervention. Tools to both enhance and measure the process should be routinely incorporated in clinical trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Graham Ferrier and Faisal Mehmud are employed by Pfizer at the time of review and writing of the manuscript. Alessandra di Pietro is employed by Pfizer at the time of review and writing of the manuscript, and also owns stocks as part of her compensation. Deepali Mittal, Geetanjali Kamath, and Saifuddin Kharawala are employees of Bridge Medical Consulting, commissioned by Pfizer to carry out this literature review. Harpreet Wasan has acted on advisory boards or as an invited speaker or has attended meetings for Servier, Pierre Fabre, Incyte, Bayer, Pfizer, Zymeworks, Merck KGaA, Taiho, Seagen, Exact therapeutics, Takeda (Hutchinson Med), Amgen, Roche/Genentech/ FM, SIRTEX Medical, Erytech Pharma, BMS (Celgene), and BTG; has acted in a consultancy or advisory capacity for NICE/BSI expert Takeda, Bayer, Pierre Fabre, ONCOSIL, Incyte, Celgene, Oaktree life sciences; has served on Global Trials committees for Pfizer (Array); Zymeworks, Boehringer Ingelheim (DMC), SIRTEX, Merck KGaA, ARCAD (Pancreas Academic); and has received research funding from MSD, Merck Serono, Pfizer, and Sirtex. Aleksandra Filipovic has no competing interests.
Figures
Similar articles
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
-
Digital interventions for the management of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD013246. doi: 10.1002/14651858.CD013246.pub2. Cochrane Database Syst Rev. 2021. PMID: 33871065 Free PMC article.
-
Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. PMID: 32129963 Free Books & Documents. Review.
-
Behavioural interventions for type 2 diabetes: an evidence-based analysis.Ont Health Technol Assess Ser. 2009;9(21):1-45. Epub 2009 Oct 1. Ont Health Technol Assess Ser. 2009. PMID: 23074526 Free PMC article.
-
Self-management support interventions for persons with chronic disease: an evidence-based analysis.Ont Health Technol Assess Ser. 2013 Sep 1;13(9):1-60. eCollection 2013. Ont Health Technol Assess Ser. 2013. PMID: 24194800 Free PMC article. Review.
References
-
- Di Maio M, Basch E, Denis F, Fallowfield LJ, Ganz PA, Howell D, et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann Oncol. 2022;33:878–92. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

