Global evolution of inflammatory bowel disease across epidemiologic stages
- PMID: 40307548
- PMCID: PMC12158780
- DOI: 10.1038/s41586-025-08940-0
Global evolution of inflammatory bowel disease across epidemiologic stages
Abstract
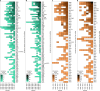
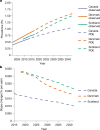
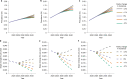
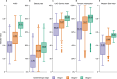
During the twentieth century, inflammatory bowel disease (IBD) was considered a disease of early industrialized regions in North America, Europe and Oceania1. At the turn of the twenty-first century, IBD incidence increased in newly industrialized and emerging regions in Africa, Asia and Latin America, while the prevalence in early industrialized regions continued to grow steadily2-4. Changes in the incidence and prevalence denote the evolution of IBD across four epidemiologic stages: stage 1 (emergence), characterized by low incidence and prevalence; stage 2 (acceleration in incidence), marked by rapidly rising incidence and low prevalence; and stage 3 (compounding prevalence), where the incidence decelerates, plateaus or declines while the prevalence steadily increases. A fourth stage (prevalence equilibrium) has been proposed in which the prevalence slope plateaus due to demographic shifts in an ageing IBD population, but it has not yet been evidenced. To date, these stages have remained theoretical, lacking specific numerical indicators to define transition points. Here, using real-world data from 522 population-based studies encompassing 82 global regions and spanning more than a century (1920-2024), we show spatiotemporal transitions across stages 1-3 and model stage 4 progression. Understanding the evolution of IBD across epidemiologic stages enables healthcare systems to better anticipate the future worldwide burden of IBD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.T.A. has received research funding from the National Institute of Health, Department of Defense, The Leona M. and Harry B. Helmsley Charitable Trust (through the University of Miami), Crohn’s and Colitis Foundation and Kenneth Rainin Foundation; she is a consultant or served on advisory boards for AbbVie, Arena Pharmaceuticals (now Pfizer), Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly and Company, Gilead Sciences, Janssen Pharmaceuticals, Janssen Global Services, Pfizer Pharmaceutical, Prometheus Biosciences, UCB Biopharma; she has received fees for lecturing from Alimentiv, Janssen Pharmaceuticals, Prime CME and WebMD Global LLC. D.B. has received speaking fees from AbbVie, Takeda and Janssen, and consulting fees from AbbVie, Takeda, Janssen and Amgen. R.B. has received grants/research support from the Asian Healthcare Foundation, Pfizer Global, Indian council of Medical Research (ICMR) and the Leona M and Harry B Helmsley Charitable Trust; and advisory board fees from Abbott, AstraZeneca, Cadila, Cipla, Celltrion, Dr Reddy Labs, Emcure, Ferring Pharmaceuticals, Hetero Drugs, Janssen, La Renon, MSN Labs, Mankind Pharma, Menarini, Micro Labs, Nestle, Pfizer, RPG Lifesciences, Sun Pharmaceuticals, Systopic, Takeda Pharmaceuticals, Torrent, Waterley and Zydus. E.I.B. has acted as a consultant for McKesson Canada and the Dairy Farmers of Ontario for matters unrelated to medications used to treat IBD; he has also acted as a consultant for the Canadian Agency for Drugs and Technology in Health (CADTH). C.N.B. is supported by the Bingham Chair in Gastroenterology; has served on advisory boards for AbbVie Canada, Amgen Canada, Bristol Myers Squibb Canada, Eli Lilly Canada, JAMP Pharmaceuticals, Janssen Canada, Pendopharm Canada, Sandoz Canada, Takeda Canada and Pfizer Canada; Consultant for Takeda; Educational grants from AbbVie Canada, Bristol Myers Squibb Canada, Ferring Canada, Pfizer Canada, Takeda Canada, Janssen Canada, Organon Canada, Eli Lilly Canada and Amgen Canada; speaker’s panel for AbbVie Canada, Janssen Canada, Pfizer Canada and Takeda Canada. Received research funding from AbbVie Canada, Amgen Canada, Sandoz Canada, Takeda Canada and Pfizer Canada. E.B.-M. has served as a speaker and consultant for Janssen, Chiesi, Takeda and Kern. J.B. reports grants and personal fees from AbbVie, grants and personal fees from Janssen-Cilag, personal fees from Celgene, grants and personal fees from MSD, personal fees from Pfizer, grants and personal fees from Takeda, grants and personal fees from Tillots Pharma, personal fees from Samsung Bioepis, grants and personal fees from Bristol Myers Squibb, grants from Novo Nordisk, personal fees from Pharmacosmos, personal fees from Ferring and personal fees from Galapagos; outside the submitted work. I.D. received consultation fees/served in advisory boards/speakers’ bureaus for AbbVie, Athos, Arena, Celltrion, Celgene/BMS, Eli-Lilly, Ferring, Food Industries Organization, Gilead, Galapagos, Iterative Scopes, Janssen, Neopharm, Pfizer, Roche/Genentech, Sangamo, Sublimity, Sandoz, Takeda, Wildbio and Prometheus. Jill Roberts IBD Center, shareholder: Gutreat, Harp diagnostics. S.E.O. has received honoraria for speaking from AbbVie and Janssen. A. Forss has served as a speaker and advisory board member for Janssen and Tillotts Pharma. R.G. has received research funding from AbbVie, Janssen, Zespri, Comvita, Goodman Fielder and Takeda; he has served on advisory boards for AbbVie, Janssen, Zespri, Comvita and Takeda; and has received honoraria from AbbVie, Janssen, Zespri and Takeda. J.L.H. has received consultation and speaking fee from Janssen, Ferring, AbbVie and Takeda. I.H. has received consulting and speaking honoraria from AbbVie, Janssen, Ferring, LF Asia, Pfizer and Takeda. B.I. has been member of advisory boards for AbbVie, Janssen and Takeda. G.-R.J. is funded by a Wellcome Trust Clinical Research Career Development Fellowship and has received speaker fees from AbbVie, Ferring Pharmaceuticals, Janssen, Takeda, Fresenius Kabi and Pfizer. F.J.-B. has received consulting and speaking fees from AbbVie, Janssen, Pfizer, Celltrion and Takeda. J.K. has served on advisory boards for Janssen Kazakhstan and has been a consultant for Takeda and Ferring; has been on speaker panels for Janssen, Takeda, Ferring, Thermo Fisher Scientific; and has received research funding from Ferring Kazakhstan. G.G.K. has received honoraria for speaking or consultancy from AbbVie, Amgen, Janssen, Pfizer, Sandoz and Pendophram; received grants for research from Ferring and for educational activities from AbbVie, Bristol Myers Squibb, Ferring, Fresenius-Kabi, Janssen, Pfizer, Takeda; he shares ownership of a patent: Treatment of inflammatory disorders, autoimmune disease and PBC; UTI Limited Partnership, assignee; patent WO2019046959A1, PCT/CA2018/051098; 7 September 2018. T.K. has served as an advisory board member, consultant or speaker for AbbVie, Activaid, Ajinomoto Bio-Pharma, Alfresa Pharma, Alimentiv, Astellas Pharma, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eisai, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen Pharmaceuticals, JIMRO, JMDC, Kissei Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda, Thermo Fisher Scientific and Zeria Pharmaceutical, and has received research funding from AbbVie, Alfresa Pharma, EA Pharma, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Sekisui Medical, Takeda, Thermo Fisher Scientific and Zeria Pharmaceutical. P.G.K. has received consulting and speaking honorarium from AbbVie, Janssen, Pfizer and Takeda. He also has received scientific grants from Pfizer and Takeda. P.L.L. has been a speaker and/or advisory board member for AbbVie, Amgen, BioJamp, Bristol Myers Squibb, Fresenius Kabi, Genetech, Gilead, Janssen, Merck, Mylan, Organon, Pendopharm, Pfizer, Roche, Sandoz, Takeda, Tillots and Viatris; and has received unrestricted research grants from AbbVie, Gilead, Takeda and Pfizer. C.W.L. is funded by a UK Research and Innovation Future Leaders Fellowship and has acted as a speaker and/or consultant to AbbVie, Janssen, Takeda, Pfizer, Galapagos, GSK, Gilead, Vifor Pharma, Ferring, Dr Falk, BMS, Boehringer Ingelheim, Novartis, Sandoz, Celltrion, Cellgene, Amgen, Samsung Bioepis, Fresenius Kabi, Tillotts, Trellus Health and Iterative Health. B.L. has received speaking fees from Tillotts Pharma and Janssen Cilag; consultancy fees from Tillotts Pharma and Bristol Myers Squib; and has received unrestricted research grants from Janssen Cilag, Tillotts Pharma; outside the submitted work. E.V.L. has consulted for AbbVie, Alvotech, Amgen, Arena, Astellas, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iota Biosciences, Iterative Health, Janssen, Morphic, Ono, Protagonist, Sun, Surrozen, Takeda, TR1X and UCB; has received research support from AbbVie, AstraZeneca, Bristol-Myers Squibb, Celgene/Receptos, Genentech, Gilead, Gossamer Bio, Janssen, Takeda, Theravance and UCB; and is a shareowner of Exact Sciences. S.C.N. has served as an advisory board member for Pfizer, Ferring, Janssen and AbbVie and received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie and Takeda; has received research grants through her affiliated institutions from Olympus, Ferring and AbbVie; is a founder member, non-executive director, non-executive scientific advisor and shareholder of GenieBiome Ltd; and receives patent royalties through her affiliated institutions. S.O. has received speaking fees from Mitsubishi Tanabe Pharma and consulting fees from EA Pharma. O.O. has been PI on projects at Karolinska Institutet partly financed by investigator-initiated grants from Janssen, Takeda, AbbVie, Pfizer, Bristoll Myers Squibb and Ferring, and also report grants from Pfizer, AbbVie, Janssen and Galapagos in the context of a national safety monitoring programs; none of these studies have any relation to the present study. The Karolinska Institutet has received fees for lectures and participation on advisory boards by O.O. from Janssen, Ferring, Takeda and Pfizer on topics not related to the present study. R.P. has received consulting fees from Abbott, AbbVie, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pendopharm, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Shire, Sublimity Therapeutics and Takeda. A.B.Q. has received speaking honorarium from AbbVie, Apsen and Janssen. R.A.R.A. has received speaking and consulting honoraria from AbbVie, Astra Zeneca, Ferring, Pfizer, Janssen and Takeda. D.T.R. has received grant support from Takeda; and has served as a consultant for AbbVie, Altrubio, Abivax SA, Altrubio, Avalo Therapeutics, Bausch Health, Bristol-Myers Squibb, Buhlmann Diagnostics, ClostraBio, Connect BioPharma, Douglas Pharmaceuticals, Eli Lilly, Genentech, InDex Pharmaceuticals, Iterative Health, Janssen Pharmaceuticals, Odyssey Therapeutics, Pfizer, Sanofi and Takeda Pharmaceuticals; he serves on the board of trustees for the Crohn’s & Colitis Foundation and is on the board of directors for Cornerstones Health. H.S. has received honoraria for speaking from Astellas, AbbVie, Biofermin, EA Pharma, Janssen, Kissei, Miyarisan, Otsuka, Tanabe-Mitsubishi, Takeda, Tsumura and Viatris; and received grants for research from Toh-so and Biofermin. M.T. has received consulting and speaking fees from AbbVie, Janssen and Takeda. D.T. received consultation fees, research grants, royalties or honoraria from Janssen, Pfizer, Shaare Zedek Medical Center, Hospital for Sick Children, Ferring, AbbVie, Takeda, Prometheus Biosciences, Lilly, Roche, Thermo Fisher Scientific, BMS and SorrisoPharma. S.C.W. has received consultancy fees from AbbVie, Bristol-Myers Squibb, Celltrion, Ferring Pharmaceuticals, Janssen, Pfizer, Takeda and Tanabe, and speaker fees from AbbVie, Bristol-Myers Squibb, Celltrion, Excelsior Biopharma, Ferring Pharmaceuticals, Pfizer, Janssen, Sanofi, Takeda and Tanabe. T.W. received funding for educational activities from AbbVie and Pfizer. J.K.Y.-F. is a speaker bureau member for AbbVie, Alfasigma, Asofarma, Bristol Myers Squibb, Carnot, Celltrion, Chinoin, Farmasa, Ferring, Janssen, Siegfried Rhein and Takeda; is a clinical research investigator Bristol Myers Squibb and Takeda; has been member of advisory boards for AbbVie, Celltrion, Ferring, Janssen and Takeda; has received research grants from Takeda; received payments for lectures from AbbVie, Alfasigma, Asofarma, Bristol Myers Squibb, Carnot, Celltrion, Chinoin, Farmasa, Ferring, Janssen, Siegfried Rhein and Takeda; and is an advisory board member of the AbbVie, Celltrion, Ferring, Janssen and Takeda. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

