Evaluation of the white test effectiveness for the prevention of bile leakage after liver resection: multicenter randomized controlled study
- PMID: 40307662
- PMCID: PMC12420697
- DOI: 10.1007/s13304-025-02210-4
Evaluation of the white test effectiveness for the prevention of bile leakage after liver resection: multicenter randomized controlled study
Abstract
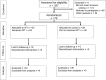
Bile leakage is a common complication after liver resection. It often requires repeated interventions or surgery and prolongs the patient's recovery. The aim of the study was to assess the effectiveness of the leakage test with fat emulsion (the White Test) in preventing postoperative biliary complications. A multicenter (3 hospitals) randomized controlled trial was performed from February 2011 to May 2016. The trial involved only the patients scheduled for major hepatectomies. After liver transection and control of biliary tree leak-proofness, the patients were randomized into two groups-with and without applying the White Test. A comparative assessment of all the White Test participants was conducted. Forty-three patients formed the study group, and 36 patients were included in the control group. The White Test revealed sites of bile leakage (the positive White Test) in 37.2% (16/43) of the patients in the study group. These leakage sites were sealed intraoperatively. One of those patients (6.2%; 1/16) still developed bile leakage after surgery. Bile leakage was still observed in 7.4% (2/27) of patients after the negative White test. The incidence of postoperatively revealed bile leakage in the study and control groups did not have a statistically significant difference: 7% (3/43) and 8.3% (3/36), respectively. All bile leaks were grade B. This study demonstrated that the White Test did not provide any benefit in preventing postoperative bile leakage; therefore, other methods, such as ICG, should be further investigated.
Keywords: Bile leakage; Complications; Liver resection; Randomized trial; White test.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: All the authors hereby declare that they have no conflicts of interest to disclose. Ethical approval: The Institution Review Board approved the study 13.05.2010 (reference number 5). Informed consent: All patients or their parents/guardians (for children) gave written consent to be included in the trial.
Figures
References
-
- Chardarov NK, Bagmet NN, Skipenko OG (2010) Biliary complications after liver resections. Khirurgiia (Mosk) 8:61–68 ([in Russian]) - PubMed
-
- Yoshioka R, Saiura A, Koga R, Seki M, Kishi Y, Yamamoto J (2011) Predictive factors for bile leakage after hepatectomy: analysis of 505 consecutive patients. World J Surg 35(8):1898–1903 - PubMed
-
- Kotelnikova LP, Grebenkina SV, Trushnikov DV (2018) Bile leakage after liver resection. Exp Clin Gastroenterol 156(8):99–106
-
- Chardarov NK, Bagmet NN, Polishchuk LO, Shatveryan GA (2010) Skipenko OG Risk factors of biliary complications after liver resections. Annaly Khirurgicheskoy Gepatologii 15(3):76–83 ([in Russian]) - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical