Clostridium scindens: history and current outlook for a keystone species in the mammalian gut involved in bile acid and steroid metabolism
- PMID: 40307670
- PMCID: PMC12065433
- DOI: 10.1093/femsre/fuaf016
Clostridium scindens: history and current outlook for a keystone species in the mammalian gut involved in bile acid and steroid metabolism
Abstract
Clostridium scindens is a keystone bacterial species in the mammalian gut that, while low in abundance, has a significant impact on bile acid and steroid metabolism. Numerous studies indicate that the two most studied strains of C. scindens (i.e. ATCC 35704 and VPI 12708) are important for a myriad of physiological processes in the host. We focus on both historical and current microbiological and molecular biology work on the Hylemon-Björkhem pathway and the steroid-17,20-desmolase pathway that were first discovered in C. scindens. Our most recent analysis now calls into question whether strains currently defined as C. scindens represent two separate taxonomic groups. Future directions include developing genetic tools to further explore the physiological role of bile acid and steroid metabolism by strains of C. scindens and the causal role of these pathways in host physiology and disease.
Keywords: Clostridium scindens; 7α-dehydroxylation; Hylemon–Björkhem pathway; gut microbiome; secondary bile acids; steroids; sterolbiome.
© The Author(s) 2025. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures






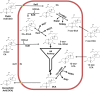

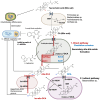
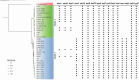
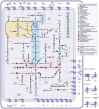
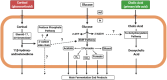
Update of
-
Clostridium scindens : an endocrine keystone species in the mammalian gut.bioRxiv [Preprint]. 2024 Aug 24:2024.08.23.609444. doi: 10.1101/2024.08.23.609444. bioRxiv. 2024. Update in: FEMS Microbiol Rev. 2025 Jan 14;49:fuaf016. doi: 10.1093/femsre/fuaf016. PMID: 39229245 Free PMC article. Updated. Preprint.
References
-
- Aigner A, Gross R, Schmid R et al. Novel 12 alpha-hydroxysteroid dehydrogenases, production and use thereof. Google Patents. 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

