Inhibition of VEGF signaling prevents exhaustion and enhances anti-leukemia efficacy of CAR-T cells via Wnt/β-catenin pathway
- PMID: 40307793
- PMCID: PMC12044824
- DOI: 10.1186/s12967-024-05907-z
Inhibition of VEGF signaling prevents exhaustion and enhances anti-leukemia efficacy of CAR-T cells via Wnt/β-catenin pathway
Abstract
Background: Current challenges in Chimeric Antigen Receptor (CAR) -T cell therapy for hematological cancers include T cell exhaustion and limited persistence, which contribute to cancer relapse.
Methods: The effects of Axitinib, a VEGFR inhibitor, on the biological functions of CAR-T cells in vitro and in vivo were investigated by comparing CAR-T cells pre-treated ex vivo with Axitinib, as well as utilizing a B-ALL mouse model. Real-time quantitative PCR and Western blotting were employed to detect the expression of molecules related to differentiation, exhaustion, and the Wnt pathway in CAR-T cells. Flow cytometry was used to assess changes in CAR-T cell differentiation, exhaustion, activation, apoptosis, proliferation, and cytokine secretion. Western blotting and flow cytometry were used to assess changes in VEGFR expression. Bioluminescence imaging, flow cytometry, and immunohistochemistry (IHC) analysis were used to evaluate changes in tumor burden in mice receiving different treatments, while hematoxylin and eosin (H&E) staining were used to monitor histological changes in the liver and spleen of mice.
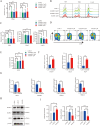
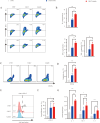
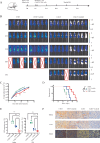
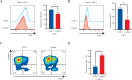
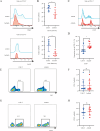
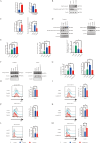
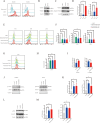
Results: Axitinib treatment notably reduced CAR-T cell exhaustion and terminal differentiation both under tonic signaling and tumor antigen exposure scenarios. Furthermore, CAR-T cells pretreated with Axitinib demonstrated enhanced anti-tumor efficacy and prolonged survival in vivo. Mechanistically, Axitinib treatment upregulated the Wnt/β-catenin signaling pathway in CAR-T cells. Using agonists/inhibitors of the Wnt/β-catenin pathway could respectively mimic or counteract the effects of Axitinib on CAR-T cell exhaustion and differentiation. CAR-T cells treated with Axitinib can inhibit the VEGFR2 pathway. CAR-T cells treated with anti-VEGFR2 antibody can activate the Wnt/β-catenin pathway and prevent CAR-T cell exhaustion.
Conclusion: Axitinib confers resistance to exhaustion in CAR-T cells by modulating the Wnt/β-catenin signaling pathway.
Keywords: Acute lymphoblastic leukemia; Chimeric antigen receptor T cells; Differentiation; Exhaustion; Vascular endothelial growth factor receptor.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was authorized and supervised by the Ethics Committee of the Zhujiang Hospital of Southern Medical University. All participants provided written informed consent before enrollment. Animal experiments were conducted in line with the Guide for the Care and Use of Laboratory Animals. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

