Hemoglobin-loaded hollow mesoporous carbon-gold nanocomposites enhance microwave ablation through hypoxia relief
- PMID: 40307855
- PMCID: PMC12042322
- DOI: 10.1186/s12951-025-03387-x
Hemoglobin-loaded hollow mesoporous carbon-gold nanocomposites enhance microwave ablation through hypoxia relief
Abstract
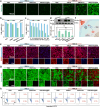
Microwave ablation, as a critical minimally invasive technique for tumor treatment, remains challenging in achieving an optimal balance between incomplete and excessive ablation. In addition to selectively elevating the temperature of tumor lesions through the microwave thermal effect, microwave-responsive nanoparticles can also improve the efficacy of single-session ablation by generating reactive oxygen species (ROS) via the microwave dynamic effect, thereby mitigating the thermal damage to normal tissues caused by high temperature. In this study, ultra-small gold nanoparticles anchored hollow mesoporous carbon nanoparticles (HMCNs) are loaded with hemoglobin (Hb) to serve as microwave ablation nano-sensitizers (HMCN/Au@Hb), which will amplify the microwave dynamic effect by alleviating the hypoxic microenvironment of tumors. Upon microwave irradiation, HMCN/Au@Hb not only improves the microwave-thermal conversion efficiency of tumor lesion but also promotes the ROS generation by increasing oxygen content in the hypoxic tumor microenvironment. More importantly, we found that the hypoxia relief will improve the antitumor response and further enhance the clearance of residual tumor after ablation. Nearly complete ablation was achieved in certain tumor-bearing mice, with no recurrence of the primary tumor observed up to 33 days post-ablation. In comparison to traditional microwave ablation, the survival time of the tumor-bearing mice was significantly extended. Therefore, this work presents an innovative ablation sensitization strategy based on the hypoxia relief and provides a nanosensitizer for microwave ablation integrating great microwave-thermal and dynamic effects along with immune modulation capabilities.
Keywords: Antitumor immune response; Hypoxia relief; Microwave ablation; Microwave dynamic effect; Nanoparticles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All the experiments were performed under protocols approved by the Animal Research Ethics Committee of Guangxi university (ethics approval number: GXU-2024-270). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aube C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–25. - PubMed
-
- Li L, Zhang X, Zhou J, Zhang L, Xue J, Tao W. Non-Invasive thermal therapy for tissue engineering and regenerative medicine. Small. 2022;18:e2107705. - PubMed
-
- Cao XJ, Wang SR, Che Y, Liu J, Cong ZB, He JF, Wang HL, Liu G, Guo JQ, Hao Y, et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: A multicenter retrospective study. Radiology. 2021;300:209–16. - PubMed
-
- Zhou H, Fu C, Chen X, Tan L, Yu J, Wu Q, Su L, Huang Z, Cao F, Ren X, et al. Mitochondria-targeted zirconium metal-organic frameworks for enhancing the efficacy of microwave thermal therapy against tumors. Biomater Sci. 2018;6:1535–45. - PubMed
-
- Ma X, Ren X, Guo X, Fu C, Wu Q, Tan L, Li H, Zhang W, Chen X, Zhong H, Meng X. Multifunctional iron-based Metal-Organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy. Biomaterials. 2019;214:119223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

