Targeted degradation of extracellular proteins: state of the art and diversity of degrader designs
- PMID: 40307925
- PMCID: PMC12044797
- DOI: 10.1186/s13045-025-01703-4
Targeted degradation of extracellular proteins: state of the art and diversity of degrader designs
Abstract
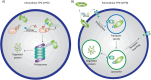
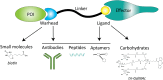
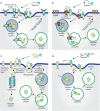
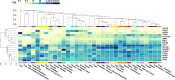
Selective elimination of proteins associated with the pathogenesis of diseases is an emerging therapeutic modality with distinct advantages over traditional inhibitor-based approaches. This strategy, called targeted protein degradation (TPD), is based on hijacking the cellular proteolytic machinery using chimeric degrader molecules that physically link the target protein of interest with the degradation effectors. The TPD era began with the development of PROteolysis TAtrgeting Chimeras (PROTACs) in 2001, with various methods and applications currently available. Classical PROTAC molecules are heterobifunctional chimeras linking target proteins with E3 ubiquitin ligases. This induced interaction leads to the ubiquitylation of the target protein, which is needed for its recognition and subsequent degradation by the cellular proteasomes. However, this technology is limited to intracellular proteins since the effectors involved (E3 ubiquitin ligases and proteasomes) are located in the cytosol. The related methods for selective destruction of proteins present in the extracellular space have only emerged recently and are collectively termed extracellular TPD (eTPD). The prototypic eTPD technology utilizes LYsosomal TArgeting Chimeras (LYTACs) that link extracellular target proteins (secreted or membrane-associated) to lysosome-targeting receptors (LTRs) on the cell surface. The resulting complex is then internalized by endocytosis and trafficked to lysosomes, where the target protein is degraded. The successful elimination of various extracellular proteins via LYTACs and related approaches has been reported, including several important targets in oncology that drive tumor growth and dissemination. This review summarizes current progress in the eTPD field and focuses primarily on the respective technological developments. It discusses the design principles and diversity of degrader molecules and the landscape of available targets and effectors that can be employed for eTPD. Finally, it emphasizes current open questions, challenges, and perspectives of this technological platform to promote the expansion of the eTPD toolkit and further development of its therapeutic applications.
Keywords: LYTAC; Membrane proteins; PROTAC; Secreted proteins; Targeted protein degradation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: The content of this manuscript has not been previously published and is not under consideration for publication elsewhere. All the authors agree to the content of the paper and their being listed as a co-author of the paper. Competing interests: The authors declare no competing interests.
Figures
References
-
- Tsai JM, Nowak RP, Ebert BL, Fischer ES. Targeted protein degradation: from mechanisms to clinic. Nat Rev Mol Cell Biol. 2024;25:740–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

