Antibody-Drug Conjugates (ADCs): current and future biopharmaceuticals
- PMID: 40307936
- PMCID: PMC12044742
- DOI: 10.1186/s13045-025-01704-3
Antibody-Drug Conjugates (ADCs): current and future biopharmaceuticals
Abstract
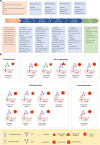
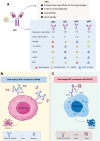
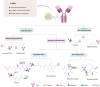
Antibody-drug conjugates (ADCs) represent a novel class of biopharmaceuticals comprising monoclonal antibodies covalently conjugated to cytotoxic agents via engineered chemical linkers. This combination enables targeted delivery of cytotoxic agents to tumor site through recognizing target antigens by antibody while minimizing off-target effects on healthy tissues. Clinically, ADCs overcome the limitations of traditional chemotherapy, which lacks target specificity, and enhance the therapeutic efficacy of monoclonal antibodies, providing higher efficacy and fewer toxicity anti-tumor biopharmaceuticals. ADCs have ushered in a new era of targeted cancer therapy, with 15 drugs currently approved for clinical use. Additionally, ADCs are being investigated as potential therapeutic candidates for autoimmune diseases, persistent bacterial infections, and other challenging indications. Despite their therapeutic benefits, the development and application of ADCs face significant challenges, including antibody immunogenicity, linker instability, and inadequate control over the release of cytotoxic agent. How can ADCs be designed to be safer and more efficient? What is the future development direction of ADCs? This review provides a comprehensive overview of ADCs, summarizing the structural and functional characteristics of the three core components, antibody, linker, and payload. Furthermore, we systematically assess the advancements and challenges associated with the 15 approved ADCs in cancer therapy, while also exploring the future directions and ongoing challenges. We hope that this work will provide valuable insights into the design and optimization of next-generation ADCs for wider clinical applications.
Keywords: Antibody–drug conjugates; Clinical application; Cytotoxic agents; Linkers; Monoclonal antibody; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Bray F, Laversanne M, Sung HYA, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Yang YQ, Wang SH, Ma PW, Jiang YL, Cheng KM, Yu Y, et al. Drug conjugate-based anticancer therapy-current status and perspectives. Cancer Lett. 2023;552:215969. - PubMed
-
- Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332(6162):323–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- No. U20A20413, China/NSFC-Liaoning joint fund key program
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- 2023JH2/20200126/Liaoning Province Scientific Research Foundation
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
- NSFC, No. 81903658, 82272797, 82304564, China/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

