Eligibility for Lecanemab Treatment in the Republic of Korea: Real-World Data From Memory Clinics
- PMID: 40308013
- PMCID: PMC12056135
- DOI: 10.3988/jcn.2024.0550
Eligibility for Lecanemab Treatment in the Republic of Korea: Real-World Data From Memory Clinics
Abstract
Background and purpose: We aimed to determine the proportion of Korean patients with early Alzheimer's disease (AD) who are eligible to receive lecanemab based on the United States Appropriate Use Recommendations (US AUR), and also identify the barriers to this treatment.
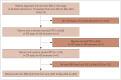
Methods: We retrospectively enrolled 6,132 patients with amnestic mild cognitive impairment or mild amnestic dementia at 13 hospitals from June 2023 to May 2024. Among them, 2,058 patients underwent amyloid positron emission tomography (PET) and 1,199 (58.3%) of these patients were amyloid-positive on PET. We excluded 732 patients who did not undergo brain magnetic resonance imaging between June 2023 and May 2024. Finally, 467 patients were included in the present study.
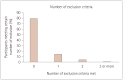
Results: When applying the criteria of the US AUR, approximately 50% of patients with early AD were eligible to receive lecanemab treatment. Among the 467 included patients, 36.8% did not meet the inclusion criterion of a Mini-Mental State Examination (MMSE) score of ≥22.
Conclusions: Eligibility for lecanemab treatment was not restricted to Korean patients with early AD except for those with an MMSE score of ≥22. The MMSE criteria should therefore be reconsidered in areas with a higher proportion of older people, who tend to have lower levels of education.
Keywords: Alzheimer's disease; Korean; Mini-Mental State Examination; eligibility; lecanemab.
Copyright © 2025 Korean Neurological Association.
Conflict of interest statement
Seong-Ho Koh, a contributing editor of the
Figures
Similar articles
-
Investigating patient eligibility for anti-amyloid monoclonal antibody treatment of Alzheimer's disease: real-world data from an Austrian psychiatric memory clinic population.BJPsych Open. 2024 Sep 23;10(5):e160. doi: 10.1192/bjo.2024.747. BJPsych Open. 2024. PMID: 39308280 Free PMC article.
-
Projected Annual Lecanemab Treatment Eligibility in an Irish Regional Specialist Memory Clinic.Int J Geriatr Psychiatry. 2024 Oct;39(10):e6157. doi: 10.1002/gps.6157. Int J Geriatr Psychiatry. 2024. PMID: 39384333
-
Lecanemab: Appropriate Use Recommendations.J Prev Alzheimers Dis. 2023;10(3):362-377. doi: 10.14283/jpad.2023.30. J Prev Alzheimers Dis. 2023. PMID: 37357276 Free PMC article.
-
Lecanemab: Appropriate Use Recommendations by Korean Dementia Association.Dement Neurocogn Disord. 2024 Oct;23(4):165-187. doi: 10.12779/dnd.2024.23.4.165. Epub 2024 Oct 29. Dement Neurocogn Disord. 2024. PMID: 39512702 Free PMC article. Review.
-
Safety and efficacy of lecanemab for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials.Front Aging Neurosci. 2023 May 5;15:1169499. doi: 10.3389/fnagi.2023.1169499. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37213538 Free PMC article.
References
-
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. - PubMed
-
- GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23:973–1003. - PubMed
-
- Booth A, Deng K. Japanese colonialism in comparative perspective. J World Hist. 2017;28:61–98.
Grants and funding
- CRC22013-600/National Research Council of Science and Technology (NST) Aging Convergence Research Center/Korea
- 2022-0-00448/RS-2022-II220448/Institute of Information and Communications Technology Planning and Evaluation/Korea
- NRF-2020M3E5D2A01084721/NRF/National Research Foundation of Korea/Korea
- RS-2021-KH112636/KDRC/Korea Dementia Research Center/Korea
- RS-2024-00337993/KDRC/Korea Dementia Research Center/Korea
LinkOut - more resources
Full Text Sources

