Integrated Multiomics Analyses of the Molecular Landscape of Sarcopenia in Alcohol-Related Liver Disease
- PMID: 40308032
- PMCID: PMC12044136
- DOI: 10.1002/jcsm.13818
Integrated Multiomics Analyses of the Molecular Landscape of Sarcopenia in Alcohol-Related Liver Disease
Abstract
Background: Skeletal muscle is a major target for ethanol-induced perturbations, leading to sarcopenia in alcohol-related liver disease (ALD). The complex interactions and pathways involved in adaptive and maladaptive responses to ethanol in skeletal muscle are not well understood. Unlike hypothesis-driven experiments, an integrated multiomics-experimental validation approach provides a comprehensive view of these interactions.
Methods: We performed multiomics analyses with experimental validation to identify novel regulatory mechanisms of sarcopenia in ALD. Studies were done in a comprehensive array of models including ethanol-treated (ET) murine and human-induced pluripotent stem cell-derived myotubes (hiPSCm), skeletal muscle from a mouse model of ALD (mALD) and human patients with alcohol-related cirrhosis and controls. We generated 13 untargeted datasets, including chromatin accessibility (assay for transposase accessible chromatin), RNA sequencing, proteomics, phosphoproteomics, acetylomics and metabolomics, and conducted integrated multiomics analyses using UpSet plots and feature extraction. Key findings were validated using immunoblots, redox measurements (NAD+/NADH ratio), imaging and senescence-associated molecular phenotype (SAMP) assays. Mechanistic studies included mitochondrial-targeted Lactobacillus brevis NADH oxidase (MitoLbNOX) to increase redox ratio and MitoTempo as a mitochondrial free radical scavenger.
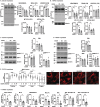
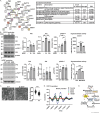
Results: Multiomics analyses revealed enrichment in mitochondrial oxidative function, protein synthesis and senescence pathways consistent with the known effects of hypoxia-inducible factor 1α (HIF1α) during normoxia. Across preclinical and clinical models, HIF1α targets (n = 32 genes) and signalling genes (n > 100 genes) (n = 3 ATACseq, n = 65 phosphoproteomics, n = 10 acetylomics, n = 6 C2C12 proteomics, n = 106 C2C12 RNAseq, n = 64 hiPSC RNAseq, n = 30 hiPSC proteomics, n = 3 mouse proteomics, n = 25 mouse RNAseq, n = 8 human RNAseq, n = 3 human proteomics) were increased. Stabilization of HIF1α (C2C12, 6hEtOH 0.24 ± 0.09; p = 0.043; mALD 0.32 ± 0.074; p = 0.005; data shown as mean difference ± standard error mean) was accompanied by enrichment in the early transient and late change clusters, -log(p-value) = 1.5-3.8, of the HIF1α signalling pathway. Redox ratio was reduced in ET myotubes (C2C12: 15512 ± 872.1, p < 0.001) and mALD muscle, with decreased expression of electron transport chain components (CI-V, p < 0.05) and Sirt3 (C2C12: 0.067 ± 0.023, p = 0.025; mALD: 0.41 ± 0.12, p = 0.013). Acetylation of mitochondrial proteins was increased in both models (C2C12: 107364 ± 4558, p = 0.03; mALD: 40036 ± 18 987, p = 0.049). Ethanol-induced SAMP was observed across models (P16: C2C12: 0.2845 ± 0.1145, p < 0.05; hiPSCm: 0.2591, p = 0.041). MitoLbNOX treatment reversed redox imbalance, HIF1α stabilization, global acetylation and myostatin expression (p < 0.05).
Conclusions: An integrated multiomics approach, combined with experimental validation, identifies HIF1α stabilization and accelerated post-mitotic senescence as novel mechanisms of sarcopenia in ALD. These findings show the complex molecular interactions leading to mitochondrial dysfunction and progressive sarcopenia in ALD.
Keywords: alcohol‐related liver disease; hypoxia‐inducible factor‐1‐alpha; mitochondrial oxidative dysfunction; protein acetylation; redox ratio; sarcopenia; senescence; sirtuins.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Grants and funding
- R56HL141744/National Institutes of Health, United States
- R56 HL141744/HL/NHLBI NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- U2C-DK119886/National Institutes of Health, United States
- 5U01DK062470-17S2/National Institutes of Health, United States
- R01 AA021890/National Institutes of Health, United States
- U01 AA 026976/National Institutes of Health, United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- 3U01AA026976-03S1/National Institutes of Health, United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- OT2-OD030544/National Institutes of Health, United States
- S10 OD030398/OD/NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- S10OD030398/National Institutes of Health, United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- S10 OD023436/OD/NIH HHS/United States
- R21 AR071046/AR/NIAMS NIH HHS/United States
- NIH K08 AA028794/National Institutes of Health, United States
- R21 AR 071046/National Institutes of Health, United States
- U01 AA021890/AA/NIAAA NIH HHS/United States
- American College of Gastroenterology Clinical Research Award
- OT2 OD030544/OD/NIH HHS/United States
- 1S10OD023436/National Institutes of Health, United States
LinkOut - more resources
Full Text Sources
Research Materials

