Efficacy and safety of immune checkpoint inhibitors for brain metastases of non-small cell lung cancer: a systematic review and network meta-analysis
- PMID: 40308503
- PMCID: PMC12040931
- DOI: 10.3389/fonc.2025.1513774
Efficacy and safety of immune checkpoint inhibitors for brain metastases of non-small cell lung cancer: a systematic review and network meta-analysis
Abstract
Background: Previous studies have demonstrated that immune checkpoint inhibitors (ICIs) significantly improve prognosis in lung cancer patients with brain metastases (BMs). This systematic review and network meta-analysis aims to evaluate the efficacy and safety of 10 ICIs recommended by the 2024 Chinese Society of Clinical Oncology guidelines for treating non-small cell lung cancer (NSCLC) without driver genes, focusing on NSCLC patients presenting with BMs.
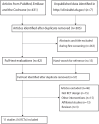
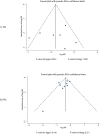
Materials and methods: A comprehensive literature search of PubMed, Embase, and the Cochrane Library was conducted through June 2024 to identify eligible controlled trials and head-to-head randomized controlled trials investigating 10 ICIs in NSCLC patients with BMs. Pairwise and network meta-analyses were performed using hazard ratios (HRs) and relative risks (RRs) with 95% confidence intervals (CIs). Treatment efficacy was ranked hierarchically through the surface under the cumulative ranking curve (SUCRA).
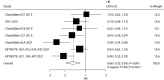
Results: Sixteen trials from 11 studies, encompassing 1,274 NSCLC patients with BMs, were included. The meta-analysis demonstrated that ICIs significantly improved overall survival (OS: HR, 0.66; 95% CI, 0.52-0.85; P = 0.001) and progression-free survival (PFS: HR, 0.67; 95% CI, 0.54-0.84; P < 0.001). SUCRA ranking identified pembrolizumab as the most effective agent for OS improvement (SUCRA 71%), while camrelizumab showed superior PFS benefits (SUCRA 92%). ICIs were associated with increased objective response rates (RR: 1.52; 95% CI, 1.13-2.06; P = 0.006), but elevated risks of immune-mediated adverse events (RR: 2.50; 95% CI, 1.46-4.30; P = 0.001) and grade 3-5 immune-mediated adverse events and infusion reaction (RR: 6.39; 95% CI, 1.53-26.69; P = 0.011).
Conclusion: ICIs demonstrate superior survival benefits compared to chemotherapy in NSCLC patients with BMs, with pembrolizumab and camrelizumab emerging as optimal choices for OS and PFS improvement, respectively. However, vigilant monitoring of immune-mediated adverse events and infusion reactions remains critical in clinical practice.
Keywords: brain metastases; immune checkpoint inhibitors; network meta-analysis; non-small cell lung cancer; systematic review.
Copyright © 2025 Liu, Chen and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

