Supersulfide donors and their therapeutic targets in inflammatory diseases
- PMID: 40308575
- PMCID: PMC12040673
- DOI: 10.3389/fimmu.2025.1581385
Supersulfide donors and their therapeutic targets in inflammatory diseases
Abstract
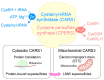
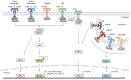
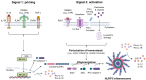
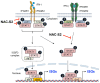
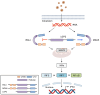
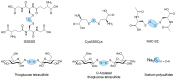
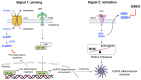
Inflammation is one defense mechanism of the body that has multiple origins, ranging from physical agents to infectious agents including viruses and bacteria. The resolution of inflammation has emerged as a critical endogenous process that protects host tissues from prolonged or excessive inflammation, which can become chronic. Failure of the inflammation resolution is a key pathological mechanism that drives the progression of numerous inflammatory diseases. Owing to the various side effects of currently available drugs to control inflammation, novel therapeutic agents that can prevent or suppress inflammation are needed. Supersulfides are highly reactive and biologically potent molecules that function as antioxidants, redox regulators, and modulators of cell signaling. The catenation state of individual sulfur atoms endows supersulfides with unique biological activities. Great strides have recently been made in achieving a molecular understanding of these sulfur species, which participate in various physiological and pathological pathways. This review mainly focuses on the anti-inflammatory effects of supersulfides. The review starts with an overview of supersulfide biology and highlights the roles of supersulfides in both immune and inflammatory responses. The various donors used to generate supersulfides are assessed as research tools and potential therapeutic agents. Deeper understanding of the molecular and cellular bases of supersulfide-driven biology can help guide the development of innovative therapeutic strategies to prevent and treat diseases associated with various immune and inflammatory responses.
Keywords: hydropersulfides; hydropolysulfides; inflammation; inflammatory responses; persulfides; polysulfides; supersulfide donors; supersulfides.
Copyright © 2025 Zhang, Pan, Sawa, Akaike and Matsunaga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

