Gui-zhi-fu-ling-wan alleviates bleomycin-induced pulmonary fibrosis through inhibiting epithelial-mesenchymal transition and ferroptosis
- PMID: 40308766
- PMCID: PMC12041222
- DOI: 10.3389/fphar.2025.1552251
Gui-zhi-fu-ling-wan alleviates bleomycin-induced pulmonary fibrosis through inhibiting epithelial-mesenchymal transition and ferroptosis
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) has a higher morbidity and poor prognosis. Gui-Zhi-Fu-Ling-Wan (GFW) is a traditional Chinese herbal formula which exerts anti-inflammatory and anti-oxidative effects. The goal was to determine the protective effect of GFW on bleomycin (BLM)-induced pulmonary fibrosis.
Methods: One hundred and twenty-four mice were randomly divided into eight groups, and orally supplemented with GFW (1 g/kg) in 1 week ago and continuing to 1 week later of single BLM intratracheal injection (5.0 mg/kg). Lung tissues were collected in 7 days and 21 days after BLM injection. BEAS-2B cells were pretreated with GFW (100 μg/mL) for three consecutive days before BLM (10 μg/mL) exposure. Cells were harvested in 12 or 24 h after BLM co-culture.
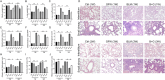
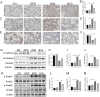
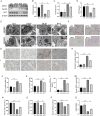
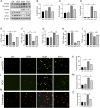
Results: GFW supplementation alleviated BLM-induced alveolar structure destruction and inflammatory cell infiltration in mice lungs. BLM-incurred collagen deposition was attenuated by GFW. In addition, GFW pretreatment repressed BLM-evoked downregulation of E-cadherin, and elevation of N-cadherin and Vimentin in mouse lungs. Besides, BLM-excited GPX4 reduction, ferritin increases, lipid peroxidation, and free iron overload were significantly relieved by GFW pretreatment in mouse lungs and BEAS-2B cells. Notably, BLM-provoked mitochondrial reactive oxygen species (mtROS) excessive production, elevation of mitochondrial stress markers, such as HSP70 and CLPP, and mitochondrial injury, were all abolished in mouse lungs and BEAS-2B cells by GFW pretreatment.
Conclusion: GFW supplementation attenuated BLM-evoked lung injury and pulmonary fibrosis partially through repressing EMT and mtROS-mediated ferroptosis in pulmonary epithelial cells.
Keywords: bleomycin; epithelial-mesenchymal transition; ferroptosis; gui-zhi-fu-ling-wan; mitochondrial reactive oxygen species; pulmonary fibrosis.
Copyright © 2025 Chen, Ma, Wang, Zhang, Xie, Wang, Guo, Liu, Cao, He, Fu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

