Association of long-term use of dipeptidyl peptidase-4 inhibitors with the risk of diabetic retinopathy in patients with diabetes mellitus: a real-world evidence study
- PMID: 40308768
- PMCID: PMC12040959
- DOI: 10.3389/fphar.2025.1518545
Association of long-term use of dipeptidyl peptidase-4 inhibitors with the risk of diabetic retinopathy in patients with diabetes mellitus: a real-world evidence study
Abstract
Background: In this study, we investigated the association of long-term use of a dipeptidyl peptidase-4 inhibitor (DPP-4i) with the risk of diabetic retinopathy (DR) in patients with diabetes mellitus (DM).
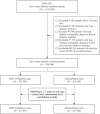
Methods: This study was a secondary analysis based on the nationwide database from 2008 to 2022 in Taiwan. Patients with new-onset DM who were treated with either a DPP-4i or sulfonylurea from 2009 to 2017 were included in this study. Patients who received a DPP-4i were included in the case group and further divided on the basis of the cumulative defined daily dose (cDDD) as follows: ≤90, 91-180, 181-300, and >300 cDDD. Propensity score matching was performed to select patients who used a sulfonylurea, and these patients were assigned to the control group. With adjustment for sex, age, income, urbanization level, comorbidities, and other anti-diabetic agents, the Cox proportional hazard model was used to estimate the risk of DR associated with DPP-4i use over the 5-year follow-up.
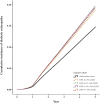
Results: There were 83,503 patients with DPP-4i use and 167,006 patients with sulfonylurea use after matching. Compared with patients with sulfonylurea use, patients with DPP-4i use at ≤90 cDDD had a hazard ratio (HR) of 1.43 (95% confidence interval [CI] = 1.38-1.49) for DR development, whereas those with DPP-4i use at 91-180, 181-300 or >300 cDDD had HRs of 1.66 (95% CI: 1.59-1.74), 1.82 (95% CI: 1.74-1.90), and 2.32 (95% CI: 1.91-2.82) for DR development, respectively. Of the different DPP-4is, linagliptin at ≤90 or 181-300 was associated with the highest risk of DR. Significant differences were discovered at ≤90, 91-181, and 181-300 cDDD in the risk of DR between patients using Saxagliptin versus sitagliptin. Vildagliptin at ≤90 or 91-180 cDDD was associated with an increased risk of DR, but not at 181-300 cDDD.
Conclusion: In patients with DM, DPP-4i at ≤90, 91-180, 181-300 and >300 cDDD was linked to an increased risk of DR over the 5-year follow-up. Sitagliptin at cDDD 181-300 was associated with the greatest DR risk. The potential for DPP-4i to accelerate DR progression should be considered.
Keywords: cumulative defined daily dose; diabetes mellitus; diabetic retinopathy; dipeptidyl peptidase-4 inhibitors; real-world evidence.
Copyright © 2025 Li, Kuan, Yang, Gau, Su, Tsai, Huang and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
LinkOut - more resources
Full Text Sources