Duodenal mucosal ablation with irreversible electroporation reduces liver lipids in rats with non-alcoholic fatty liver disease
- PMID: 40308802
- PMCID: PMC12038522
- DOI: 10.3748/wjg.v31.i16.105188
Duodenal mucosal ablation with irreversible electroporation reduces liver lipids in rats with non-alcoholic fatty liver disease
Abstract
Background: Duodenal mucosal ablation (DMA) using irreversible electroporation (IRE) with a glucagon-like peptide-1 receptor agonist has been clinically shown to reduce liver lipid deposition in non-alcoholic fatty liver disease (NAFLD). However, the specific metabolic contributions of DMA using IRE in NAFLD remain unclear.
Aim: To assess the feasibility and effectiveness of DMA using IRE in NAFLD rat models.
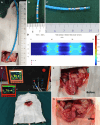
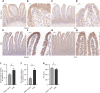
Methods: Seven-week-old male Sprague-Dawley rats underwent DMA using IRE after 8 weeks on a high-fat diet. Two weeks post-treatment, duodenal and liver tissues and blood samples were collected. We evaluated differences in the duodenal wall structure, liver lipid deposition, enteroendocrine, claudin, and zonula ocludens-1 in the duodenal mucosa.
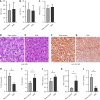
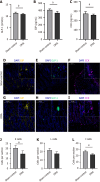
Results: DMA using IRE could be safely performed in rats with NAFLD without duodenal bleeding, perforation, or stenosis. The duodenum healed well 2 weeks after DMA and was characterized by slimmer villi, narrower and shallower crypts, and thicker myenterons compared with the sham-control setting. Liver lipid deposition was reduced and serum lipid index parameters were considerably improved in the DMA setting. However, these improvements were independent of food intake and weight loss. In addition, enteroendocrine parameters, such as claudin, and zonula ocludens-1 levels in the duodenal mucosa, differed between the different settings in the DMA group.
Conclusion: By altering enteroendocrine and duodenal permeability, simple DMA using IRE ameliorated liver lipid deposition and improved serum lipid parameters in NAFLD rats.
Keywords: Duodenal mucosal ablation; Duodenal permeability; Enteroendocrine; Irreversible electroporation; Non-alcoholic fatty liver disease.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


Similar articles
-
Endoscopic duodenal mucosa ablation techniques for diabetes and nonalcoholic fatty liver disease: A systematic review.Med. 2024 Jul 12;5(7):735-758.e2. doi: 10.1016/j.medj.2024.03.014. Epub 2024 Apr 4. Med. 2024. PMID: 38579730
-
Duodenal mucosal resurfacing: proof-of-concept, procedural development, and initial implementation in the clinical setting.Gastrointest Endosc. 2019 Oct;90(4):673-681.e2. doi: 10.1016/j.gie.2019.03.024. Epub 2019 Mar 29. Gastrointest Endosc. 2019. PMID: 30935932
-
Duodenal-jejunal bypass improves nonalcoholic fatty liver disease independently of weight loss in rodents with diet-induced obesity.Am J Physiol Gastrointest Liver Physiol. 2020 Oct 1;319(4):G502-G511. doi: 10.1152/ajpgi.00357.2019. Epub 2020 Aug 19. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32812775
-
Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease.World J Gastroenterol. 2019 Sep 14;25(34):5105-5119. doi: 10.3748/wjg.v25.i34.5105. World J Gastroenterol. 2019. PMID: 31558860 Free PMC article.
-
Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats.Sci Rep. 2017 Mar 16;7:44819. doi: 10.1038/srep44819. Sci Rep. 2017. PMID: 28300221 Free PMC article.
Cited by
-
Duodenal mucosal ablation: An emerging therapeutic concept for metabolic dysfunction-associated fatty liver disease.World J Gastroenterol. 2025 Jul 28;31(28):109468. doi: 10.3748/wjg.v31.i28.109468. World J Gastroenterol. 2025. PMID: 40741478 Free PMC article.
References
-
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. - PubMed
-
- Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–530. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

