Stem cell therapy for intervertebral disc degeneration: Clinical progress with exosomes and gene vectors
- PMID: 40308883
- PMCID: PMC12038459
- DOI: 10.4252/wjsc.v17.i4.102945
Stem cell therapy for intervertebral disc degeneration: Clinical progress with exosomes and gene vectors
Abstract
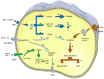
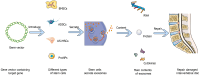
Intervertebral disc degeneration is a leading cause of lower back pain and is characterized by pathological processes such as nucleus pulposus cell apoptosis, extracellular matrix imbalance, and annulus fibrosus rupture. These pathological changes result in disc height loss and functional decline, potentially leading to disc herniation. This comprehensive review aimed to address the current challenges in intervertebral disc degeneration treatment by evaluating the regenerative potential of stem cell-based therapies, with a particular focus on emerging technologies such as exosomes and gene vector systems. Through mechanisms such as differentiation, paracrine effects, and immunomodulation, stem cells facilitate extracellular matrix repair and reduce nucleus pulposus cell apoptosis. Despite recent advancements, clinical applications are hindered by challenges such as hypoxic disc environments and immune rejection. By analyzing recent preclinical and clinical findings, this review provided insights into optimizing stem cell therapy to overcome these obstacles and highlighted future directions in the field.
Keywords: Exosomes; Extracellular matrix repair; Gene vector system; Hypoxic environment; Intervertebral disc degeneration; Mesenchymal stem cells; Regenerative medicine; Stem cell therapy.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
References
-
- Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398–408. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

