RNA interference-mediated osteoprotegerin silencing increases the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio and promotes osteoclastogenesis
- PMID: 40308885
- PMCID: PMC12038464
- DOI: 10.4252/wjsc.v17.i4.101290
RNA interference-mediated osteoprotegerin silencing increases the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio and promotes osteoclastogenesis
Abstract
Background: In vivo degradation of bone scaffolds is significantly influenced by osteoclast (OC) activity, which is orchestrated by the interplay between receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin (OPG). The ratio of RANKL/OPG is a crucial determinant of OC-mediated bone resorption, which plays an integral role in bone remodeling and scaffold degradation. Elevated levels of RANKL relative to OPG enhance osteoclastogenesis, thereby accelerating the degradation process essential for integrating bone scaffolds into the host tissue.
Aim: To elucidate the effects of OPG gene silencing on osteoclastogenesis within rat bone marrow-derived mesenchymal stem cells (BMSCs). By investigating these effects, the study aimed to provide deeper insights into the regulatory mechanisms that influence bone scaffold degradation, potentially leading to improved bone repair and regeneration strategies.
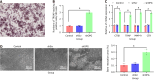
Methods: We employed recombinant lentiviral plasmids to silence the OPG gene in rat BMSCs to achieve the aims. The efficacy of gene silencing was assessed using quantitative reverse transcription polymerase chain reaction and western blot analysis to measure the expression levels of OPG and RANKL. Tartrate-resistant acid phosphatase staining was utilized to evaluate the formation of OCs. Additionally, co-immunoprecipitation assays were conducted to explore the interactions between RANKL and OPG proteins, further assessing the biochemical pathways involved in osteoclastogenesis.
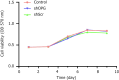
Results: The silencing of the OPG gene in BMSCs resulted in a significant increase in the RANKL/OPG ratio, evidenced by decreased expression levels of OPG and increased levels of RANKL. Enhanced osteoclastogenesis was observed through tartrate-resistant acid phosphatase staining, which indicated a substantial rise in OC formation in response to the altered RANKL/OPG balance. The co-immunoprecipitation assays provided concrete evidence of the direct interaction between RANKL and OPG proteins, substantiating their pivotal roles in regulating OC activity.
Conclusion: The findings from this study underscore the critical role of the RANKL/OPG axis in osteoclastogenesis. Silencing of the OPG gene in BMSCs effectively increases the RANKL/OPG ratio, promoting OC activity and potentially enhancing bone scaffold degradation. This regulatory mechanism offers a promising avenue for modulating bone remodeling processes, which is essential for effective bone repair and the successful integration of bone scaffolds into damaged sites. Future research might focus on optimizing the control of this axis to better facilitate bone tissue engineering and regenerative therapies.
Keywords: Bone marrow-derived mesenchymal stem cells; Bone scaffold; Osteoclast; Osteoprotegerin; RNA interference; Receptor activator of nuclear factor-kappa B ligand.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures






References
-
- Dos Santos Jorge Sousa K, de Souza A, de Almeida Cruz M, de Lima LE, do Espirito Santo G, Amaral GO, Granito RN, Renno AC. 3D printed scaffolds of biosilica and spongin from marine sponges: analysis of genotoxicity and cytotoxicity for bone tissue repair. Bioprocess Biosyst Eng. 2024;47:1483–1498. - PubMed
-
- Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021;39:2–11. - PubMed
LinkOut - more resources
Full Text Sources

