Application of metal stable isotopes labeling and elemental mass spectrometry for biomacromolecule profiling
- PMID: 40308936
- PMCID: PMC12035746
- DOI: 10.52601/bpr.2024.240039
Application of metal stable isotopes labeling and elemental mass spectrometry for biomacromolecule profiling
Abstract
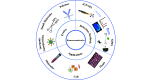
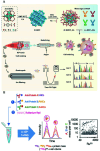
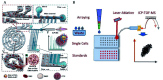
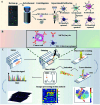
Biomacromolecules including proteins and nucleic acids are widely recognized for their pivotal and irreplaceable role in maintaining the normal functions of biological systems. By combining metal stable isotope labeling with elemental mass spectrometry, researchers can quantify the amount and track the spatial distribution of specific biomacromolecules in complex biological systems. In this review, the probes classification and metal stable isotope labeling strategies are initially summarized. Secondly, the technical characteristics and working principle of the elemental mass spectrometry techniques including inductively coupled plasma mass spectrometry and secondary ion mass spectrometry are introduced to achieve highly sensitive detection of multiple biomacromolecules at molecular, cellular and tissue levels. Lastly, we underline the advantages and limitations of elemental mass spectrometry combined with metal stable isotope labeling strategies, and propose the perspectives for future developments.
Keywords: Biomacromolecule; Mass spectrometry analysis; Metal stable isotope labeling.
© The Author(s) 2025.
Conflict of interest statement
Ping Zhang, Ying Han, Yue Xu and Liang Gao declare that they have no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
