Post-coordination of Ru(ii) controlled regioselective B(4)-H acylmethylation of o-carboranes with sulfoxonium ylides
- PMID: 40308949
- PMCID: PMC12038429
- DOI: 10.1039/d5sc01576f
Post-coordination of Ru(ii) controlled regioselective B(4)-H acylmethylation of o-carboranes with sulfoxonium ylides
Abstract
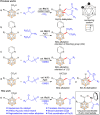
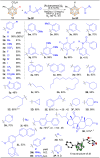
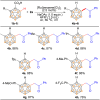
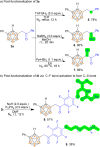
Despite significant progress in the B-H functionalization of carboranes, the development of cost-effective catalytic systems devoid of noble metals, coupled with mechanistic validation of regioselectivity control, remains a formidable challenge. Herein, we disclose an Ag salt-free, redox-neutral, and inexpensive ruthenium(ii)-catalyzed protocol that enables exclusive B(4)-H acylmethylation of o-carboranes through a novel post-coordination strategy. By exploiting weakly coordinating carboxylic acid as a traceless directing group, this method achieves excellent mono-site selectivity for B-C(sp3) bond formation using diverse sulfoxonium ylides, demonstrating both functional group tolerance and synthetic scalability. This work not only establishes a practical synthetic platform but also addresses critical mechanistic questions unresolved in prior analogous studies. Through deuterium labeling, in situ high-resolution mass spectrometry (HRMS) tracking, and single-crystal X-ray analysis of critical Ru intermediates, we unequivocally demonstrate that the mono-site selectivity originates from a unique post-coordination mode of Ru(ii). The Ru catalyst simultaneously engages both the carboxylic acid and the enolizable acylmethyl moiety in the mono-acylated intermediate, thereby dictating the B(4)-H activation trajectory. Our findings establish a generalizable platform for regiocontrolled carborane functionalization while defining mechanistic paradigms in transition metal-mediated B-H activation chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Marfavi A. Kavianpour P. Rendina L. M. Nat. Rev. Chem. 2022;6:486–504. doi: 10.1038/s41570-022-00400-x. - DOI - PubMed
- Scholz M. Hey-Hawkins E. Chem. Rev. 2011;111:7035–7062. - PubMed
- Issa F. Kassiou M. Rendina L. M. Chem. Rev. 2011;111:5701–5722. doi: 10.1021/cr2000866. - DOI - PubMed
- Grams R. J. Santos W. L. Scorei I. R. Abad-García A. Rosenblum C. A. Bita A. Cerecetto H. Viñas C. Soriano-Ursúa M. A. Chem. Rev. 2024;124:2441–2511. doi: 10.1021/acs.chemrev.3c00663. - DOI - PubMed
-
- Han Y.-F. Jin G.-X. Acc. Chem. Res. 2014;47:3571–3579. doi: 10.1021/ar500335a. - DOI - PubMed
- Popescu A. R. Teixidor F. Viñas C. Coord. Chem. Rev. 2014;269:54–84. doi: 10.1016/j.ccr.2014.02.016. - DOI
- Cui P.-F. Liu X.-R. Jin G.-X. J. Am. Chem. Soc. 2023;145:19440–19457. doi: 10.1021/jacs.3c05563. - DOI - PubMed
-
- Núñez R. Tarrés M. Ferrer-Ugalde A. de Biani F. F. Teixidor F. Chem. Rev. 2016;116:14307–14378. doi: 10.1021/acs.chemrev.6b00198. - DOI - PubMed
- Ochi J. Tanaka K. Chujo Y. Angew. Chem., Int. Ed. 2020;59:9841–9855. doi: 10.1002/anie.201916666. - DOI - PubMed
- Yuhara K. Tanaka K. Angew. Chem., Int. Ed. 2024;63:e202319712. - PubMed
- Wei X. Zhu M.-J. Cheng Z. Lee M. Yan H. Lu C. Xu J.-J. Angew. Chem., Int. Ed. 2019;58:3162–3166. doi: 10.1002/anie.201900283. - DOI - PubMed
- Tanaka K. Gon M. Ito S. Ochi J. Chujo Y. Coord. Chem. Rev. 2022;472:214779. doi: 10.1016/j.ccr.2022.214779. - DOI
- Liu K. Zhang J. Shi Q. Ding L. Liu T. Fang Y. J. Am. Chem. Soc. 2023;145:7408–7415. doi: 10.1021/jacs.2c13843. - DOI - PubMed
- Tu D. Leong P. Guo S. Yan H. Lu C. Zhao Q. Angew. Chem., Int. Ed. 2017;56:11370–11374. doi: 10.1002/anie.201703862. - DOI - PubMed
- Axtell J. C. Kirlikovali K. O. Djurovich P. I. Jung D. Nguyen V. T. Munekiyo B. Royappa A. T. Rheingold A. L. Spokoyny A. M. J. Am. Chem. Soc. 2016;138:15758–15765. doi: 10.1021/jacs.6b10232. - DOI - PubMed
- Lee Y. H. Park J. Lee J. Lee S. U. Lee M. H. J. Am. Chem. Soc. 2015;137:8018–8021. doi: 10.1021/jacs.5b04576. - DOI - PubMed
- Ma W. Zhang J. Zong J. Ren H. Tu D. Xu Q. Zhong Tang B. Yan H. Angew. Chem., Int. Ed. 2024;63:e202410430. doi: 10.1002/anie.202410430. - DOI - PubMed
- Saha A. Oleshkevich E. Vinas C. Teixidor F. Adv. Mater. 2017;29:1704238. doi: 10.1002/adma.201704238. - DOI - PubMed
- Cioran A. M. Musteti A. D. Teixidor F. Krpetić Ž. Prior I. A. He Q. Kiely C. J. Brust M. Viñas C. J. Am. Chem. Soc. 2012;134:212–221. doi: 10.1021/ja203367h. - DOI - PubMed
LinkOut - more resources
Full Text Sources

