Quinonoid radial π-conjugation
- PMID: 40308951
- PMCID: PMC12038938
- DOI: 10.1039/d5sc00639b
Quinonoid radial π-conjugation
Abstract
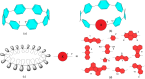
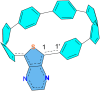
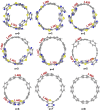
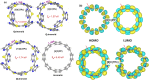
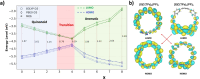
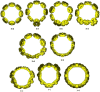
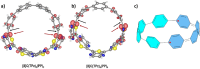
Radially π-conjugated macrocycles with mixed aromatic and quinonoid units are considered. As a function of including an increasing number of aromatic units into a ring-like nanohoop with quinonoid units, a transition occurs where the HOMO and LUMO levels cross, leading to a topological transition described for the first time. Such transitions have been seen before in ethynylene-linked oligoacene polymers as a function of the acene size on gold surfaces and in various π-conjugated polymers as a function of external strain, but not in small molecular nanohoops or any other zero-dimensional system. Near the level crossing, the HOMO-LUMO gap becomes very small, offering novel photophysical properties while maintaining extensive delocalization. The open shell character of the rings changes continuously as the composition is gradually changed, switching from a singlet ground state to a triplet, providing a zero-dimensional analogy to topological transitions between a non-trivial to a trivial phase as observed in linear one-dimensional conjugated polymers. The spins of the triplet are localized near the two aromatic-quinonoid connections.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Leonhardt E. J. Jasti R. Nat. Rev. Chem. 2019;3:672–686. doi: 10.1038/s41570-019-0140-0. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

