Tandem reaction-powered near-infrared fluorescent molecular reporter for real-time imaging of lung diseases
- PMID: 40308959
- PMCID: PMC12038431
- DOI: 10.1039/d5sc01488c
Tandem reaction-powered near-infrared fluorescent molecular reporter for real-time imaging of lung diseases
Abstract
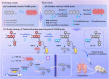
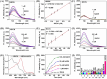
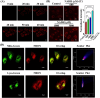
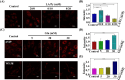
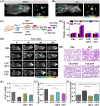
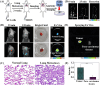
Diabetes and its complications have drawn growing research attention due to their detrimental effects on human health. Although optical probes have been used to help understand many aspects of diabetes, the lung diseases caused by diabetes remain unclear and have rarely been explored. Herein, a tandem-reaction (TR) strategy is proposed based on the adjacent diol esterification-crosslinking reaction and the nicotinamide reduction reaction of nicotinamide adenine dinucleotide (NADH) to design a lung-targeting near-infrared (NIR) small molecule probe (NBON) for accurate imaging of diabetic lung diseases. NBON was designed by coupling a phenylboronic acid analog that can form borate ester bonds by reversibly binding with NADH via an esterification-crosslinking reaction. Streptozotocin (STZ)-induced diabetic mice and metformin (MET)/epalrestat (EPS)-repaired model studies demonstrated that NBON allowed the sensitive imaging of NADH for lung disease diagnosis and therapeutic monitoring. The proposed antioxidant mechanism by which EPS alleviates diabetic lung disease was studied for the first time in living cells and in vivo. Furthermore, NBON was successfully applied in the detection of NADH in tumors and lung metastases. Overall, this work provides a general platform for a NIR NADH probe design, and advances the development of NADH probes for mechanistic studies in lung diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Schuyler M. R. Niewoehner D. E. Inkley S. R. Kohn R. Am. Rev. Respir. Dis. 1976;113:37–41. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

