The effects of cyclic peptide [R4W4] in combination with first-line therapy on the survival of Mycobacterium avium
- PMID: 40308965
- PMCID: PMC12041069
- DOI: 10.3389/fcimb.2025.1547376
The effects of cyclic peptide [R4W4] in combination with first-line therapy on the survival of Mycobacterium avium
Abstract
Background: Mycobacterium avium (M. avium) is a nontuberculous mycobacterium (NTM) that can cause pulmonary and extrapulmonary infections mostly in immunocompromised individuals, such as those with HIV and diabetes. Traditionally, rifampicin (RIF) and azithromycin (AZ) have been used for a 12-month duration as first-line antibiotics against M. avium. Due to the increased multidrug resistance, novel ways, such as enhancement of macrophages response, are needed to provide adequate immune response required to clear M. avium infection.
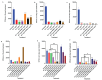
Methods and findings: In this study, we aim to study the effects of using THP-1 cells, which are monocyte-like cells, to induce a macrophage response and control M. avium infection when used in combination with traditional treatments such as RIF and AZ in free and liposomal forms. Traditional treatments' effects are studied when used alone and in combination therapy with cyclic peptide [R4W4] (liposomal encapsulated and liposomal combination). Colony-forming units (CFU) counts were assessed for all samples 3 hours, 4 days, and 8 days post-treatment. A significant reduction in the intracellular viability of M. avium was observed when THP-1 cells were treated with liposomal combination [R4W4]+RIF and liposomal combination [R4W4]+AZ compared to when treated with liposomal RIF or liposomal AZ alone, respectively.
Conclusion: Our findings show that liposomal combination [R4W4] is a promising adjuvant therapy to increase M. avium susceptibility to known antibiotics.
Keywords: Mycobacterium avium; antibiotics; antimicrobial; cyclic peptide; macrophages.
Copyright © 2025 Kelley, Sasaninia, Badaoui, Glassman, Abnousian, Rai, Tiwari and Venketaraman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

