Evaluating the Diagnostic Potential of Biomarker Panels in Breast Cancer and Prostate Adenocarcinoma
- PMID: 40309623
- PMCID: PMC12040734
- DOI: 10.1002/hsr2.70796
Evaluating the Diagnostic Potential of Biomarker Panels in Breast Cancer and Prostate Adenocarcinoma
Abstract
Background: Noninvasive diagnostic methods are essential for early cancer detection and improved patient outcomes. Circulating biomarkers, measurable indicators of pathological processes, offer a promising avenue, yet optimal panels for reliable cancer diagnosis remain undefined. This study evaluates the diagnostic performance of selected plasma biomarkers in distinguishing breast cancer and prostate adenocarcinoma patients from healthy individuals, using statistical analysis and machine learning.
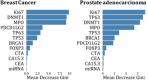
Materials and methods: We analyzed blood samples from 162 participants (73 cancer patients: 51 with breast cancer and 22 with prostate adenocarcinoma; 89 healthy controls). Levels of 12 cancer-associated biomarkers-including Ki67, DNMT1, BRCA1, and MPO-were quantified using enzyme-linked immunosorbent assays (ELISA). Statistical analyses, including the Mann-Whitney U test and machine learning models (random forest), were employed to assess the predictive accuracy of these biomarkers in distinguishing between cancerous and healthy states.
Results: Biomarkers such as Ki67, DNMT1, and MPO were significantly elevated in cancer groups. Random forest models using selected combinations (e.g., BRCA1-CTA-TP53) achieved perfect classification accuracy (AUC = 1.00). However, high inter-marker correlations suggested potential redundancy, underscoring the need for biomarker panel optimization.
Conclusion: Our findings support the potential of biomarker panels for accurate, noninvasive cancer diagnostics. Further validation in larger, more diverse cohorts is warranted to establish clinical utility and generalizability.
Keywords: biomarkers; blood samples; cancer; machine learning; panel.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A novel mixed integer programming for multi-biomarker panel identification by distinguishing malignant from benign colorectal tumors.Methods. 2015 Jul 15;83:3-17. doi: 10.1016/j.ymeth.2015.05.011. Epub 2015 May 15. Methods. 2015. PMID: 25980368
-
Integrating multi-cohort machine learning and clinical sample validation to explore peripheral blood mRNA diagnostic biomarkers for prostate cancer.Cancer Cell Int. 2025 Apr 22;25(1):158. doi: 10.1186/s12935-025-03788-w. Cancer Cell Int. 2025. PMID: 40264196 Free PMC article.
-
Multi-omics Biomarker Pipeline Reveals Elevated Levels of Protein-glutamine Gamma-glutamyltransferase 4 in Seminal Plasma of Prostate Cancer Patients.Mol Cell Proteomics. 2019 Sep;18(9):1807-1823. doi: 10.1074/mcp.RA119.001612. Epub 2019 Jun 27. Mol Cell Proteomics. 2019. PMID: 31249104 Free PMC article.
-
Diagnostic Accuracy of Blood-based Biomarkers for Pancreatic Cancer: A Systematic Review and Meta-analysis.Cancer Res Commun. 2022 Oct 20;2(10):1229-1243. doi: 10.1158/2767-9764.CRC-22-0190. eCollection 2022 Oct. Cancer Res Commun. 2022. PMID: 36969742 Free PMC article.
-
MicroRNA panels as diagnostic biomarkers for colorectal cancer: A systematic review and meta-analysis.Front Med (Lausanne). 2022 Nov 7;9:915226. doi: 10.3389/fmed.2022.915226. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36419785 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

