CXCL12+ fibroblastic reticular cells in lymph nodes facilitate immune tolerance by regulating T cell-mediated alloimmunity
- PMID: 40309773
- PMCID: PMC12043101
- DOI: 10.1172/JCI182709
CXCL12+ fibroblastic reticular cells in lymph nodes facilitate immune tolerance by regulating T cell-mediated alloimmunity
Abstract
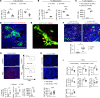
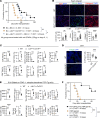
Fibroblastic reticular cells (FRCs) are the master regulators of the lymph node (LN) microenvironment. However, the role of specific FRC subsets in controlling alloimmune responses remains to be studied. Single-cell RNA sequencing (scRNA-Seq) of naive and draining LNs (DLNs) of heart-transplanted mice and human LNs revealed a specific subset of CXCL12hi FRCs that expressed high levels of lymphotoxin-β receptor (LTβR) and are enriched in the expression of immunoregulatory genes. CXCL12hi FRCs had high expression of CCL19, CCL21, indoleamine 2,3-dioxygenase (IDO), IL-10, and TGF-β1. Adoptive transfer of ex vivo-expanded FRCs resulted in their homing to LNs and induced immunosuppressive environments in DLNs to promote heart allograft acceptance. Genetic deletion of LTβR and Cxcl12 in FRCs increased alloreactivity, abrogating the effect of costimulatory blockade in prolonging heart allograft survival. As compared with WT recipients, CXCL12+ FRC-deficient recipients exhibited increased differentiation of CD4+ T cells into Th1 cells. Nano delivery of CXCL12 to DLNs improved allograft survival in heart-transplanted mice. Our study highlights the importance of DLN CXCL12hi FRCs in promoting transplant tolerance.
Keywords: Adaptive immunity; Immunology; Tolerance; Transplantation.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

