A structural element within the 5'UTR of β-catenin mRNA modulates its translation under hypoxia
- PMID: 40309781
- PMCID: PMC12044334
- DOI: 10.1093/nar/gkaf321
A structural element within the 5'UTR of β-catenin mRNA modulates its translation under hypoxia
Abstract
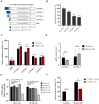
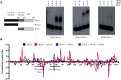
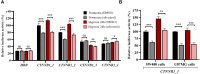
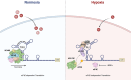
Tight regulation of translation initiation is crucial for cellular adaptation to environmental changes. Stress conditions like hypoxia trigger translational reprogramming of mRNAs encoding proteins essential for stress recovery and cell survival. Recent studies highlight alternative translation initiation pathways based on specific motifs in mRNA 5' untranslated regions (5'UTRs). Notably, β-catenin is of particular interest since maintaining its translation promotes cancer cell persistence and plasticity. β-Catenin, an oncogenic protein, plays a key role in Wnt signalling. Besides dysregulation of the β-catenin/Wnt pathway, chemotherapy-induced hypoxia leads to abnormal nuclear β-catenin accumulation, modulating gene expression linked to cancer progression and metastasis. However, the mechanism sustaining β-catenin translation in stressed cells remains elusive. To explore how β-catenin mRNA evades global translational blockade in hypoxic cancer cells, we analysed its 5'UTR and identified a translation regulatory element in cellulo. We discovered a GC-rich three-way junction (TWJ) structure within the β-catenin 5'UTR enhancing its hypoxia-driven translation. A polypurine region within the TWJ anchors eIF4B, eIF4A, and eIF4G2. Importantly, the TWJ makes β-catenin mRNA translation eIF4A-dependent and sensitive to silvestrol, a selective eIF4A inhibitor and promising anticancer agent. This study elucidates the 5'UTR-driven β-catenin mechanism under hypoxia, paving the way to inhibit its translation in cancer.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to A.S. (
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

